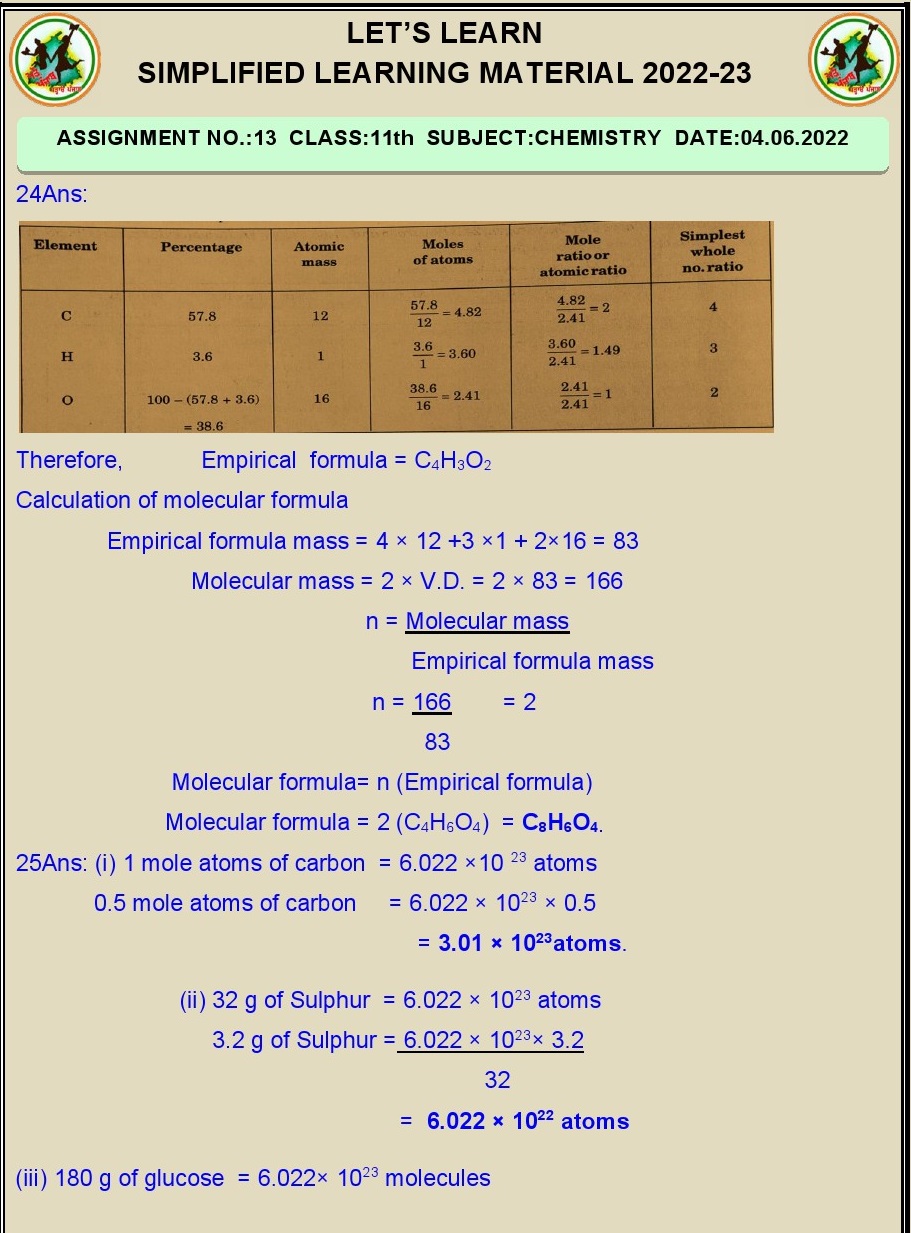
Unit 1 Some Basic Concepts Of Chemistry
VERY SHORT QUESTIONS ANSWER
Q.1.What are the number of significant
figures in 0.001620?
Ans. Four.
Q.2.The height of a person is
155.01cm.what is the least count of the scale used?
Ans.01.
Q.3.What is that S.I. unit of energy?
Ans. Joule.
Q.4.Express 5.607892 to four
significant figures and write the result in standard form.
Ans.5.608 ×106.
Q.5.Express the result of
Ans.0.561.
Q.6. Is brass a compound or a mixture?
Ans. It is a mixture.
Q.7.What are the number of significant figures in
0.002360?
Ans. Four.
Q.8.How many grams are present in 5.6 liters of CO2
at N.T.P.?
Ans. 11 grams.
Q.9.The height of a person is 163.07 cm. What is the
least count of the scale used?
Ans.0.01 cm.
Q.10. Define the term ’unit of measurement?
Ans. A unit of measurement is a standardized quantity used to
quantify and compare different attributes or properties of objects or
phenomena.
Q.11.What is the relationship in G.M.W and G.M.V?
Ans. G.M.W. and G.M.V. are usually synonymous terms, both
representing the total value of goods or services transacted through a platform
or marketplace.
Q.12.What is matter?
Ans. Matter is anything that occupies space and has mass.
Q.13.What are mixtures?
Ans. Mixtures are combinations of two or more substances that are
physically intermingled but not chemically bonded.
Q.14.What is homogeneous mixture?
Ans. A homogeneous mixture is a mixture with uniform composition,
where the components are evenly distributed and not visibly distinguishable.
Q.15.What is homogenecous mixture?
Ans. Heterogeneous these do not have uniform composition e.g.
mixtures.
Q.16.Name physical quantities which are
represented by the following units and give their most common names:
(A). kg m2s-2 (B) kg ms-2
Ans. (a) Energy, joule (b) Force, Newton.
Q.17. Is low of constant composition true for all
types of compounds?
Ans. no, the law of constant composition does not hold true for all
types of compounds.
Q.18.Which method will be used for separating a
mixture of two components having different adsorbing tendencies on an
adsorbate?
Ans. Chromatography.
Q.19. Define area (A)?
Ans. Area (A) =Length x Length =m x m = m2
Q.20.Define unit of work (W)?
Ans. The unit of work (W) is defined as the joule (J).
Q.21. Define Energy?
Ans. Energy is the ability or capacity to do work, it comes in
various forms such as kinetic, potential, thermal, chemical, electrical, etc.
and is essential for all processes and phenomena in the universe.
Q.22.What is a compound?
Ans. A compound is a substance composed of two or more different
elements chemically bonded together.
Q.23.Define Element?
Ans. An element is a pure substance composed of only one type of
atom.
Q.24.Define an organic compound Give one example?
Ans. An organic compound is a compound primarily composed of carbon
atoms bonded to hydrogen atoms and may also include other elements like oxygen,
nitrogen, etc.
Example: Ethanol (C2H5OH)
Q.25.Define an inorganic compound Give one
example?
Ans. An inorganic compound is a compound that does not primarily
contain carbon-hydrogen (C-H) bonds.
Example: Sodium chloride (NaCl)
Q.26.What does S.T.P. Stand for?
Ans. S.T.P. stands for Standard Temperature and Pressure.
Q.27.Define Avogadro’s Low?
Ans. Avogadro's Law states that equal volumes of gases at the same
temperature and pressure contain an equal number of molecules.
Q.28.What is gram atomic mass Give one example?
Ans. Gram atomic mass is the mass of one mole of atoms of an element,
expressed in grams.
Example: The gram atomic mass of carbon (C) is approximately
12.01 grams per mole.
Q.29.Define mole in terms of number?
Ans. A mole is a unit of measurement that represents Avogadro's
number (approximately 6.022 x 10^23) of particles, such as atoms, molecules,
ions, or other entities in a substance.
Q.30. Give important applications of Avogadro’s
Law?
1. Ans. Determination of molar
volume
2. Gas stoichiometry
3. Ideal Gas Law
4. Determination of molar
mass
5. Understanding gas
behavior
Q.31. Define a chemical equation?
Ans. A chemical equation is a symbolic representation of a chemical
reaction, showing the reactants on the left side and the products on the right
side, separated by an arrow.
Q.32.State the law of multiple proportion?
Ans. The law of multiple proportions states that when elements
combine to form different compounds, the ratios of the masses of one element
that combine with a fixed mass of the other element can be expressed in small
whole numbers.
Q.33. How many electrons are present in 16 g of CH4?
Ans. I molecule of CH4 = 6 + 4 = 10 electrons.
Q.34.What is the relationship between atomic
weight and equivalent weight of an element?
Ans. The relationship between the atomic weight and equivalent weight
of an element is that the equivalent weight is equal to the atomic weight
divided by the valency (or the number of electrons exchanged in a chemical
reaction).
SHORT QUESTIONS ANSWER
Q.1.What is the difference between 4.0 g and 4.00
g?
Ans. The difference between 4.0 g and 4.00 g lies in the level of precision
in their measurements.
4.0 g implies that the value has one significant figure,
indicating a precision to the tenths place (±0.1 g).
4.00 g, on the other hand, indicates a value with two
significant figures, showing precision to the hundredths place (±0.01 g).
In summary, 4.00 g has a higher level of precision compared to
4.0 g.
Q.2.Write main postulates of Dalton‘s Theory?
Ans. Dalton's Atomic Theory, proposed by John Dalton in the early
19th century, consists of the following main postulates:
Elements are composed of indivisible and indestructible
particles called atoms.
All atoms of a given element are identical in mass and
properties, while atoms of different elements have different masses and
properties.
Atoms combine in whole-number ratios to form compounds.
In chemical reactions, atoms are rearranged, but they are
neither created nor destroyed.
Atoms of different elements can combine to form compounds in fixed
ratios.
These postulates formed the foundation of modern atomic theory
and greatly contributed to our understanding of the nature of matter and
chemical reactions.
Q.3. Give the points of difference between mixture
and compound?
Ans. Points of difference between mixture and compound:
Definition:
Mixture: A mixture is a
combination of two or more substances in which each substance retains its
individual properties, and they are physically intermingled.
Compound: A compound is a substance composed of two or more
different elements chemically bonded together in fixed proportions.
Composition:
Mixture: The composition of a
mixture can vary, and the substances involved can be present in any proportion.
Compound: Compounds have a fixed
chemical composition, with elements always present in specific and definite
ratios.
Separation:
Mixture: Components of a mixture
can be separated by physical methods, such as filtration, distillation, or
chromatography.
Compound: Compounds can only be
separated into their constituent elements through chemical reactions.
Properties:
Mixture: Each component in a
mixture retains its individual properties, and the properties of a mixture are
a combination of the properties of its constituents.
Compound: Compounds have unique properties
different from those of the elements they are composed of.
Formation:
Mixture: Mixtures are formed by
physically mixing substances.
Compound: Compounds are formed by
chemical bonding between elements.
Examples:
Mixture: Air (a mixture of
gases), saltwater (a mixture of salt and water).
Compound: Water (H2O), carbon
dioxide (CO2).
In summary, mixtures are physically combined substances with
variable composition, while compounds are chemically bonded substances with
fixed composition and unique properties.
Q.4.Write some important postulates of Modern
Atomic Theory?
Ans. Some important postulates of Modern Atomic Theory include:
Atoms are
composed of subatomic particles: Atoms are no longer considered
indivisible. They are made up of subatomic particles, namely protons, neutrons,
and electrons.
Nucleus and
electron cloud model: The atom has a central nucleus containing protons and neutrons,
while electrons exist in orbits or energy levels around the nucleus.
Neutral atom
and atomic number: In a neutral atom, the number of protons is equal to the number
of electrons. The atomic number represents the number of protons in the nucleus
of an atom, defining its unique identity.
Isotopes: Atoms of the same
element can have different numbers of neutrons, resulting in isotopes with
varying atomic masses.
Electron
configuration and energy levels: Electrons occupy specific energy levels
or shells around the nucleus, and each shell can accommodate a specific number
of electrons.
Quantum
mechanics: The behavior of subatomic particles is described by quantum
mechanics, involving wave-particle duality and quantized energy levels.
Chemical
bonding: Atoms can form chemical bonds by sharing, gaining, or losing
electrons to achieve a stable electron configuration.
Conservation
of mass and energy: The total mass and energy are conserved in chemical reactions.
Atoms are rearranged, but no atoms are created or destroyed.
These postulates, based on experimental evidence and quantum
theory, form the foundation of our current understanding of atoms and their
behavior in chemistry and physics.
Q.5.Write some applications of mole concept?
Ans. The mole concept is a fundamental concept in chemistry that has
various applications, including:
Stoichiometry: The mole concept enables
stoichiometric calculations, where the quantities of reactants and products in
a chemical reaction are determined based on their molar ratios.
Balancing
chemical equations: Using the mole concept, chemical equations can be balanced by
ensuring that the same number of moles of each element are present on both
sides of the equation.
Determining
empirical and molecular formulas: The mole concept allows the determination
of empirical and molecular formulas based on the molar ratios of elements in a
compound.
Concentration
calculations: The concept of molarity (moles of solute per liter of solution)
is used to express the concentration of a solution in various chemical
processes.
Gas laws: The mole concept is
essential in the study of gas laws, such as the Ideal Gas Law, which involves
the number of moles of gas in a given volume.
Mass-mole
conversions: The mole concept facilitates conversions between the mass of a
substance and its molar quantity, aiding in various calculations in chemical
reactions.
Limiting
reactant calculations: The mole concept helps identify the limiting reactant in a
chemical reaction, which determines the maximum amount of product that can be
formed.
Percent
composition: The mole concept allows the determination of the percent
composition of elements in a compound based on their molar masses.
Overall, the mole concept is a versatile tool used to understand
and solve a wide range of chemical problems and calculations in various fields
of chemistry.
Q.6.What do you mean by limiting reagent or
limiting reactant?
Ans. The limiting reagent, also known as the limiting reactant, is a
key concept in stoichiometry that refers to the reactant that limits the amount
of product that can be formed in a chemical reaction. In other words, it is the
reactant that is completely consumed or used up during the reaction,
determining the maximum amount of product that can be produced.
When two or more reactants are involved in a chemical reaction,
they react according to their stoichiometric ratios based on their coefficients
in the balanced chemical equation. The reactant that is present in lesser molar
quantity compared to the stoichiometric ratio will be the limiting reagent.
Once this limiting reagent is consumed, the reaction stops, and any excess of
the other reactant(s) remains unreacted.
To find the limiting reagent, one can compare the amounts of
each reactant given or available and calculate how much product can be formed
from each. The reactant that yields the smallest amount of product is the
limiting reagent.
It is essential to identify the limiting reagent accurately in
order to calculate the theoretical yield (the maximum amount of product that
can be obtained) and to understand the actual yield (the amount of product
obtained in a real-world scenario). Stoichiometric calculations are based on
the amount of the limiting reagent, as it governs the reaction's outcome.
Q.7.Draw a pictorial presentation for a mole?
Ans. As an AI language model, I don't have the capability to draw
images directly. However, I can describe a simple pictorial representation of a
mole:
Imagine a group of objects, such as balls or spheres, all of the
same size and color. Each ball represents a single particle, like an atom or a
molecule. Now, gather a specific number of these balls together to form a group
or a pile.
This group of balls represents one mole of particles. The number
of balls in this pile corresponds to Avogadro's number, which is approximately
6.022 x 10^23 particles. This large quantity of particles is what a mole
represents in chemistry.
Please keep in mind that this is a simplified visual
representation to help you grasp the concept of a mole. In reality, individual
particles are too tiny to see and count individually, but Avogadro's number
represents the incredibly large scale of particles in a mole.
Q.8.What is balancing of chemical equation? On
what principle the balancing of a chemical equation is based?
Ans. Balancing a chemical equation is the process of adjusting the
coefficients of reactants and products to ensure that the same number of atoms
of each element are present on both sides of the equation. It follows the law
of conservation of mass, which states that mass is neither created nor
destroyed in a chemical reaction.
When balancing a chemical equation, the total number of atoms of
each element must be the same on both sides of the equation to maintain the
principle of mass conservation. This is because atoms cannot be created or destroyed
during a chemical reaction; they can only rearrange to form new compounds.
The steps to balance a chemical equation involve:
Write the unbalanced equation, showing the reactants on the left
and the products on the right, separated by an arrow.
Identify the number of atoms of each element present on both
sides of the equation.
Start balancing by adjusting the coefficients (whole numbers) in
front of the compounds, making sure the number of atoms of each element is the
same on both sides.
Continue adjusting coefficients until the equation is balanced,
with equal numbers of atoms of each element on both sides.
The balanced equation provides a quantitative representation of
the chemical reaction, showing the relative amounts of reactants and products
involved, based on the law of conservation of mass. It is a crucial tool for
understanding chemical reactions and performing stoichiometric calculations.
Q.9.What are the limitations of a chemical
equation how these limitations could be removed?
Ans. The limitations of a chemical equation are as follows:
Lack of
Information on Reaction Conditions: Chemical equations do not provide
information about reaction conditions such as temperature, pressure, catalysts,
or the speed of the reaction.
Incomplete
Representation: Chemical equations may not fully capture all the intermediate
steps or side reactions that occur during a complex chemical process.
No
Information on Mechanism: Chemical equations do not reveal the detailed reaction
mechanisms or the path the reaction takes to form products.
Idealized
Stoichiometry: Chemical equations assume perfect stoichiometry, but in
real-world reactions, actual yields may be different due to limitations like
side reactions, impurities, or incomplete conversions.
To remove these limitations, additional information and
techniques can be employed:
Reaction
Conditions: Provide relevant information about the reaction conditions, such
as temperature, pressure, and catalysts, to get a more comprehensive
understanding of the reaction.
Mechanism
Studies: Experimental techniques, like kinetic studies and spectroscopy,
can help reveal the detailed mechanism and intermediate steps of a reaction.
Stoichiometry
Adjustments: Use stoichiometry and stoichiometric calculations to account for
impurities or to determine the actual yield of a reaction.
Mechanistic
Studies: Conducting mechanistic studies using advanced techniques like
computational chemistry or NMR spectroscopy can provide insights into reaction
pathways and intermediates.
While chemical equations provide a useful framework to represent
reactions, these limitations can be addressed through additional experimental
data and theoretical studies, allowing for a more accurate and comprehensive
understanding of chemical processes.
Q.10. Explain the terms empirical and molecular
formulae of a compound how are they related to each other?
Ans. Empirical
Formula: The empirical formula of a compound represents the simplest
whole-number ratio of atoms of each element present in the compound. It does
not provide the actual number of atoms or the arrangement of atoms in the
molecule. The empirical formula is derived from the percentage composition or experimental
data of a compound.
Molecular
Formula: The molecular formula of a compound shows the actual number of
atoms of each element present in a single molecule of the compound. It provides
the true representation of the composition of the molecule.
Relationship
between Empirical and Molecular Formulae: The molecular formula is related to the
empirical formula through a simple integer multiple. If the compound's
molecular formula is known, the empirical formula can be determined by dividing
the subscripts of each element in the molecular formula by their greatest
common divisor (GCD).
For example, if the molecular formula of a compound is C6H12O6,
the empirical formula can be found by dividing each subscript (6, 12, 6) by
their GCD (6), resulting in the empirical formula CH2O.
On the other hand, if only the empirical formula is known, the
molecular formula can be obtained by determining the actual molar mass of the
compound and comparing it with the empirical formula's molar mass. The
molecular formula will be a whole number multiple of the empirical formula to
match the actual molar mass.
In summary, the empirical formula gives the simplest ratio of
atoms in a compound, while the molecular formula provides the actual number of
atoms in a molecule. The two are related by a whole-number multiple, allowing
the determination of one from the other given the necessary data.