CHAPTER 2 STRUCTURE OF
ATOM
VERY SHORT QUESTIONS ANSWER
Q.1.What happens when a high voltage is
passed through air in discharge tube at low pressure?
Ans. Plasma.
Q.2.How can you say cathode rays
consist of negatively charge particles?
Ans. Deflection.
Q.3.What happens when an electric
discharge is passed through a gas in discharge tube fitted with a perforated
cathode?
Ans. Glow.
Q.4.What is an electron and who
discovered it?
Ans. Subatomic. J.J. Thomson.
Q.5.What are protons and who discovered
protons?
Ans. Positive. Ernest Rutherford.
Q.6.What is neutron and who discovered
it?
Ans. Neutral. James Chadwick.
Q.7. Define atomic number?
Ans. Number of protons.
Q.8. Define mass number?
Ans. Total protons and neutrons.
Q.9.What do you understand by dual
nature of radiations?
Ans. Wave-particle duality of radiations means that
electromagnetic radiation, such as light, exhibits both wave-like and
particle-like properties simultaneously.
Q.10.What is dual nature of Electrons?
Ans. Wave-particle duality of electrons means that electrons,
like other subatomic particles, exhibit both wave-like and particle-like
properties simultaneously.
Q.11.What is photoelectric effect?
Ans. Emission.
Q.12.What are electromagnetic
radiations? Give two examples?
Ans. Light waves. Examples: Visible light and radio waves.
Q.13. Define (A) frequency (B)
wavelength?
Ans. (A) Frequency: Number of cycles per unit time, usually measured in Hertz
(Hz).
(B)
Wavelength: Distance between two
consecutive points in a wave that are in phase, typically measured in meters
(m).
Q.14. How is photon different from
proton?
Ans. Photon: Particle of electromagnetic radiation with no mass and no
electric charge.
Proton: Subatomic particle with positive charge found in the
nucleus of an atom and has mass.
Q.15.Name the element whose isotope has
mass number 14 and 8 neutrons?
Ans. Oxygen.
Q.16.State Heisenberg‘s uncertainty
principle?
Ans. Uncertainty principle: Simultaneous measurement.
Q.17. Define pauli Exclusion principle?
Ans. Exclusion: Two identical fermions.
Q.18. Define Hund‘s rule of maximum
multiplicity?
Ans. Maximum: Electrons occupy.
Q.19. Define Aufbau principle?
Ans. Aufbau: Electrons fill.
Q.20.What is the relationship between
wavelength and momentum of a particle?
Ans. Inverse.
Q.21.Which quantum number specifies the
shape of an orbital?
Ans. The quantum number that specifies the shape of an orbital
is the azimuthal quantum number, also known as the angular momentum quantum
number (l).
Q.22. By what name is the following
principle known Electrons with the same spin quantum number cannot be present
in the same atomie orbital?
Ans. Pauli Exclusion Principle.
Q.23.Which quantum number will express
the difference in two electrons in k-shell?
Ans. The magnetic quantum number (m) expresses the difference
in two electrons in the K-shell.
Q.24.Name the phenomena that indicate
the dual nature of electrons?
Ans. The phenomenon that indicates the dual nature of electrons
is the electron diffraction.
Q.25.Why can we not assign a definite
line path to a moving electron?
Ans. Wave-particle duality.
Q.26.How does change in velocity of a
moving particle alter the wavelength related to the particle?
Ans. As the velocity of a moving particle increases, its
wavelength decreases (and vice versa) according to the de Broglie wavelength
equation.
Q.27.What is the significance of
angular quantum number?
Ans. Shape of orbitals.
Q.28.Which quantum number determines
the orientation of an atomic orbital?
Ans. The magnetic quantum number determines the orientation of
an atomic orbital.
Q.29.Which quantum number is linked to
the shape of an orbital in an atom?
Ans. The azimuthal quantum number is linked to the shape of an
orbital in an atom.
Q.30.Which quantum number determines
the energy associated with an orbital in an atom?
Ans. The principal quantum number determines the energy
associated with an orbital in an atom.
Q.31.Why can the motion of an electron
around the nucleus not be determined accurately?
Ans. Due to the inherent wave-like nature of electrons in
quantum mechanics, their exact position and velocity cannot be simultaneously
determined accurately. This limitation is described by Heisenberg's Uncertainty
Principle.
Q.32. Define an atomic orbital what
does angular momentum quantum number tall about an orbital?
Ans. Atomic orbital: Region around the nucleus where electrons are likely to
be found.
Angular momentum quantum
number: Describes the shape and type of orbital (s, p, d, f) in an atom.
Q.34.Give the maximum number of
electrons which can be accommodated in a set of (A) P- orbitals and (B)
d-orbitals.
Ans. (A) P-orbitals: The maximum number of electrons that can be accommodated
in a set of P-orbitals is 6 (2 electrons in each of the three P orbitals).
(B)
D-orbitals: The maximum number of
electrons that can be accommodated in a set of D-orbitals is 10 (2 electrons in
each of the five D orbitals).
Q.35.What is the nature of VIBGYOR?
Ans. VIBGYOR refers to the colors of the visible light
spectrum. It stands for Violet, Indigo, Blue, Green, Yellow, Orange, and Red.
These are the colors that can be seen by the human eye when white light is
dispersed or refracted, such as through a prism.
Q.36. What values are permitted for the
angular momentum quantum number I for an electron with principal quantum number
, n =4?
Ans. For an electron with principal quantum number (n) = 4,
the permitted values for the angular momentum quantum number (l) can be 0, 1,
2, or 3. These correspond to the different subshells with shapes s, p, d, and
f, respectively.
Q.37.Which quantum number specifies the
energy if an electron in an atom?
Ans. The principal quantum number (n) specifies the energy of
an electron in an atom.
Q.38. Why do may elements have
fractional atomic masses?
Ans. Many elements have fractional atomic masses due to the
presence of isotopes, which have different masses and abundances, contributing
to the weighted average.
Q.39.When do electrons from various energy
levels fall to first energy level in hydrogen Name the series of spectral
lines?
Ans. Electrons from various energy levels fall to the first
energy level in hydrogen during electronic transitions, producing the Lyman
series of spectral lines.
Q.40.What is meant by quantisation of
energy?
Ans. Quantization of energy refers to the concept that energy
levels in certain physical systems are discrete and can only take specific,
quantized values, rather than having continuous values.
SHORT QUESTIONS ANSWER
Q.1.What was Thomson, s plum pudding
model of an atom?
Ans. Thomson's plum pudding model of an atom, proposed in the
early 20th century, suggested that the atom is a uniform, positively charged
sphere with negatively charged electrons embedded within it like plums in a
pudding. It implied that the negative and positive charges were evenly
distributed throughout the atom. However, this model was later replaced by the
more accurate Rutherford model.
Q.2.What do you mean by jnterference
and diffraction?
Ans. Interference and diffraction are both phenomena that
occur when waves interact with each other or with obstacles.
Interference: Interference is the phenomenon that takes place when two
or more waves combine and their amplitudes either reinforce (constructive
interference) or cancel out (destructive interference) at specific points in
space. It occurs when waves overlap, leading to a new wave pattern.
Diffraction: Diffraction is the bending or spreading of waves as they
encounter obstacles or pass through narrow openings. It occurs when waves
encounter an obstacle that is comparable in size to their wavelength, causing
the waves to change direction and spread out beyond the obstacle's edges.
Diffraction is more prominent with waves of longer wavelengths, such as sound
waves or water waves.
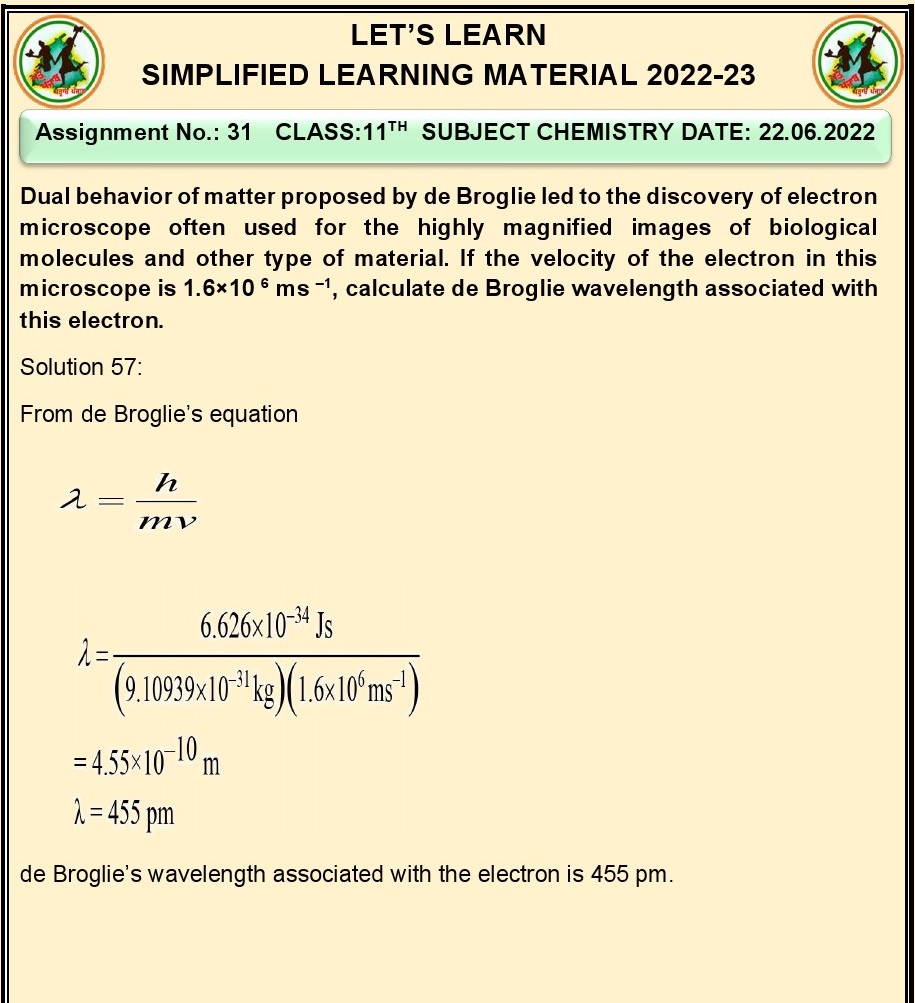
Q.3.Calculat the number of electrons
which will together weigh one gram?
Ans. To calculate the number of electrons that together weigh
one gram, we need to use the mass of a single electron and Avogadro's constant.
Mass of a single electron
(m_e) ≈ 9.109 x 10^-31 kg
Avogadro's constant (N_A) ≈
6.022 x 10^23 mol^-1
We know that 1 gram is equal
to 0.001 kg.
Now, let's find the number
of electrons:
Number of electrons = (1
gram) / (mass of a single electron)
Number of electrons = (0.001
kg) / (9.109 x 10^-31 kg)
Number of electrons ≈ 1.099
x 10^26 electrons
So, approximately 1.099 x
10^26 electrons will together weigh one gram.
Q.4.What do you mean by electromagnetic
spectrum?
Ans. The electromagnetic spectrum refers to the entire range
of electromagnetic waves, which are a form of energy that travels in the form
of waves at the speed of light. It encompasses a broad range of frequencies and
wavelengths, from the longest radio waves to the shortest gamma rays.
The electromagnetic
spectrum includes various types of waves, such as:
Radio waves
Microwaves
Infrared radiation
Visible light
Ultraviolet radiation
X-rays
Gamma rays
Each of these waves has different
properties and applications. Visible light is the portion of the
electromagnetic spectrum that can be seen by the human eye, while other types
of waves have applications in communications, imaging, heating, medical
treatments, and scientific research.
Q.5.What do you mean by Heisenberg ’s
uncertainty principle?
Ans. Heisenberg's uncertainty principle is a fundamental
concept in quantum mechanics, formulated by the German physicist Werner
Heisenberg in 1927. It states that it is impossible to simultaneously know both
the exact position (location) and the exact momentum (velocity) of a subatomic
particle, such as an electron.
In other words, the more
precisely we try to measure the position of a particle, the less precisely we
can determine its momentum, and vice versa. This principle arises from the
wave-like nature of particles at the quantum level, where their properties are
described by probability distributions rather than definite values.
Heisenberg's uncertainty
principle has profound implications for our understanding of the behavior of
subatomic particles and places limitations on the accuracy of measurements in
the microscopic world. It represents a fundamental uncertainty in our knowledge
of quantum systems and is a cornerstone of quantum mechanics.
Q.6. Why is Heisenberg’s uncertainty
principle not useful in daily life?
Ans. Heisenberg's uncertainty principle is not useful in daily
life for several reasons:
It
is applicable at the quantum level: Heisenberg's uncertainty principle applies to particles
at the atomic and subatomic scale. In our daily lives, we deal with macroscopic
objects where quantum effects are negligible.
Macroscopic
objects have well-defined properties: In everyday situations, the objects we interact with have
well-defined positions and momenta in classical physics. The uncertainty
principle becomes significant only when dealing with extremely small particles,
such as electrons and photons.
Quantum
effects are typically not observable: Quantum effects become prominent when dealing with
particles with very short wavelengths, which are far beyond our ability to
observe or manipulate in daily life.
Technological
limitations: Measuring devices and
methods in our daily lives are not sensitive enough to detect the minute
uncertainties predicted by the uncertainty principle at the quantum level.
Overall, the uncertainty
principle's effects are confined to the realm of the very small and do not have
practical implications in our daily experiences with the macroscopic world. It
remains a crucial concept for understanding quantum mechanics and the behavior
of subatomic particles in the realm of modern physics and technology.
Q.7. Give important postulates of
Planck‘s quantum theory of radiation?
Ans. Planck's quantum theory of radiation, proposed by Max
Planck in 1900, laid the foundation for quantum mechanics and explained the
behavior of blackbody radiation. The important postulates of Planck's quantum
theory are:
Energy
Quantization: Planck proposed that
the energy of electromagnetic radiation (such as light) is not continuous but
quantized. He introduced the idea of energy packets called "quanta"
or "photons," where each quantum carries an energy proportional to
its frequency (E = hf), and h is Planck's constant (approximately 6.626 x 10^-34
joule-seconds).
Oscillators
and Resonators: Planck
considered the blackbody as an ensemble of oscillators (charged particles) that
can only emit or absorb energy in discrete multiples of hf. This postulate
successfully explained the observed spectrum of blackbody radiation, which
classical theories failed to account for.
Discrete
Energy Levels: The energy levels of
these oscillators are quantized, and they can only change energy states in
discrete steps by absorbing or emitting whole-number multiples of hf.
Planck's quantum theory
marked a significant departure from classical physics and paved the way for the
development of quantum mechanics, eventually leading to a revolution in our
understanding of the behavior of particles and waves at the atomic and
subatomic scales.
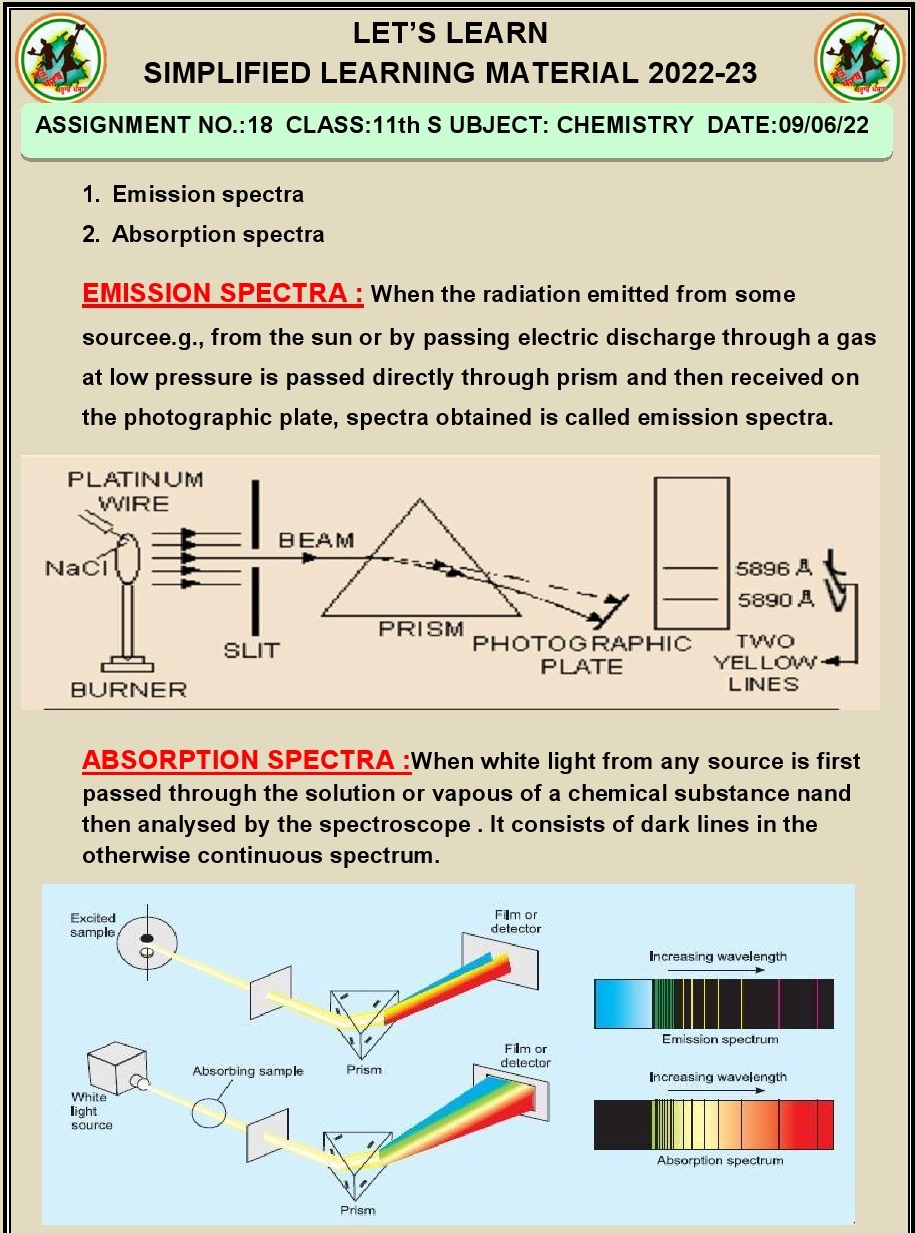
Q.8.What is emission spectra Explain
different types of emission spectra?
Ans. Emission spectra refer to the characteristic patterns of
electromagnetic radiation (light) emitted by atoms or molecules when they
transition from higher energy states to lower energy states. These transitions
occur when excited electrons within the atom or molecule lose energy and return
to their ground state.
There are three types
of emission spectra:
Continuous
Spectrum: A continuous spectrum
is produced by a source of light that emits a continuous range of wavelengths
without any gaps or lines. Incandescent light bulbs and white sunlight are
examples of sources that produce continuous spectra.
Line
Spectrum (or Bright-line Spectrum): A line spectrum consists of discrete, bright lines of
different colors separated by dark spaces. Each line corresponds to a specific
wavelength of light emitted when excited electrons in an atom or molecule
return to lower energy levels. These lines are characteristic of the elements
or molecules emitting the light and are used in spectroscopy for identification
and analysis.
Band
Spectrum (or Absorption Spectrum): A band spectrum shows a series of continuous bands of
colors with dark spaces or gaps between them. These spectra result from the
absorption of specific wavelengths of light by an atom or molecule, where
certain energy levels are allowed, and others are forbidden. The bands appear
when light passes through a medium, such as a gas, that absorbs specific
wavelengths.
The study of emission
spectra is crucial in understanding atomic and molecular structures and has
applications in various fields, including astronomy, chemistry, and physics. It
provides valuable information about the energy levels and transitions of
electrons within atoms and molecules, leading to a deeper understanding of the
properties and behavior of matter at the quantum level.
Q.9. Write at least four differences
between orbit and orbital?
Ans. Definition:
Orbit: An orbit refers to the well-defined, fixed path followed
by an electron around the nucleus in the Bohr model of the atom.
Orbital: An orbital is a probability distribution or region in
space where there is a high probability of finding an electron in the quantum
mechanical model of the atom.
Nature:
Orbit: In the Bohr model, orbits are considered as specific,
circular paths with a fixed radius and energy for electrons in an atom.
Orbital: In the quantum mechanical model, orbitals are described
as three-dimensional regions around the nucleus, characterized by specific
shapes and energy levels.
Uncertainty:
Orbit: Orbits were considered to be precisely defined paths with
no uncertainty in the electron's position or momentum.
Orbital: Orbital describes the probability of finding an electron
at a particular location, so there is inherent uncertainty in its exact
position.
Compatibility with
Quantum Mechanics:
Orbit: The Bohr model's concept of orbits was based on classical
physics and was later found to be incompatible with the principles of quantum
mechanics.
Orbital: Orbitals are a fundamental concept in quantum mechanics
and provide a more accurate description of the electron's behavior in atoms,
incorporating wave-like properties.
Q.10. Why is the energy of electron in
an atom negative?
Ans. In the context of quantum mechanics and the Schrödinger
equation, the energy of an electron in an atom is negative when it is bound to
the nucleus. This negativity arises from the nature of the mathematical
solutions to the Schrödinger equation, which describe the behavior of electrons
as wave functions.
The negative energy
indicates that the electron's energy is lower (more stable) in the bound state
within the atom compared to being completely free and far away from the
nucleus. When an electron is bound to the nucleus, it is subject to the
attractive force of the positively charged protons in the nucleus, and this
attractive force lowers the electron's potential energy.
In quantum mechanics, the
absolute value of the energy itself is not as important as the differences in
energy levels. Negative energy merely represents that the electron is in a
bound state, whereas positive energy values typically refer to free electrons
or those in higher energy states. The concept of negative energy is a
mathematical result and plays a crucial role in understanding the stability and
behavior of electrons within atoms.