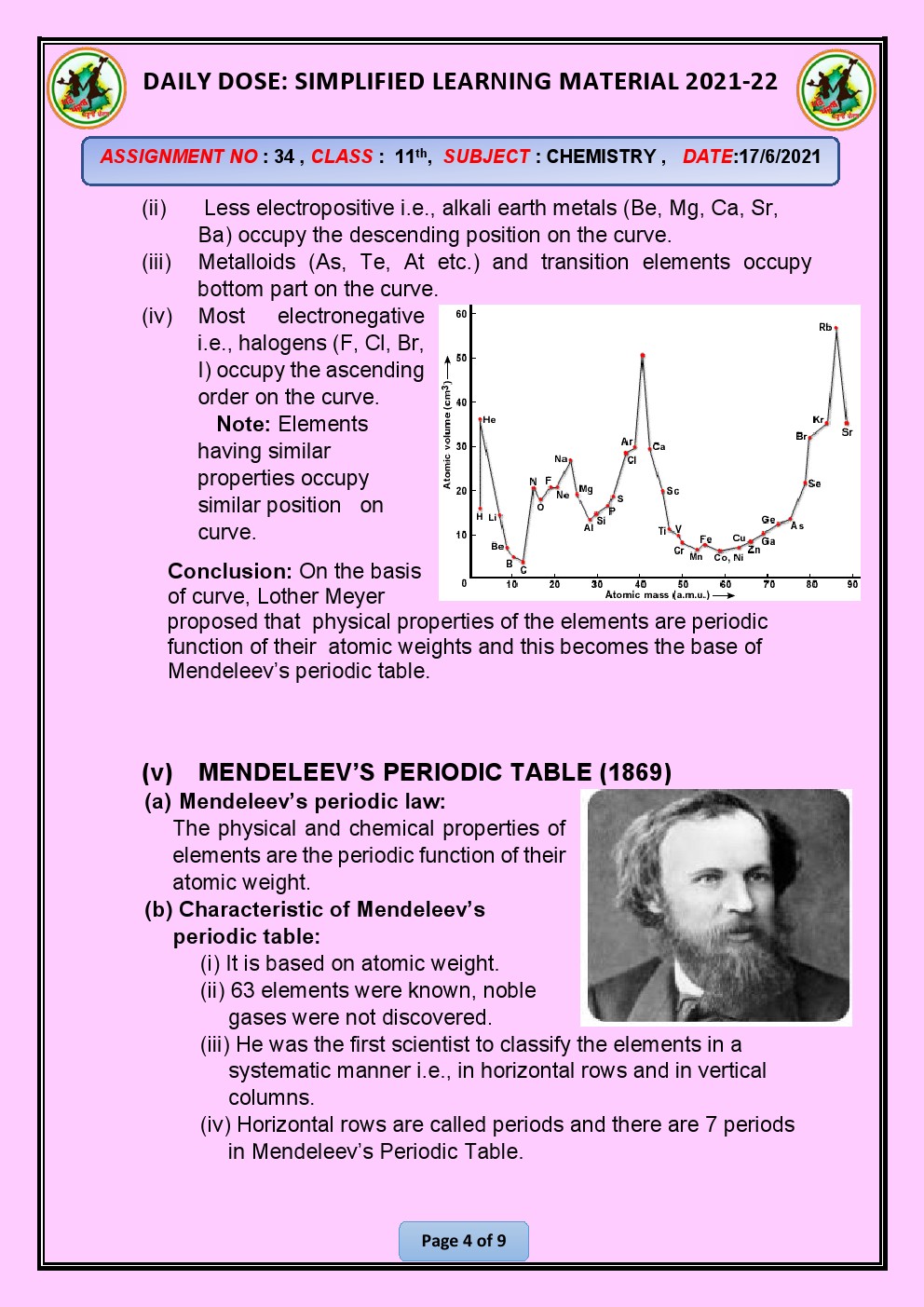
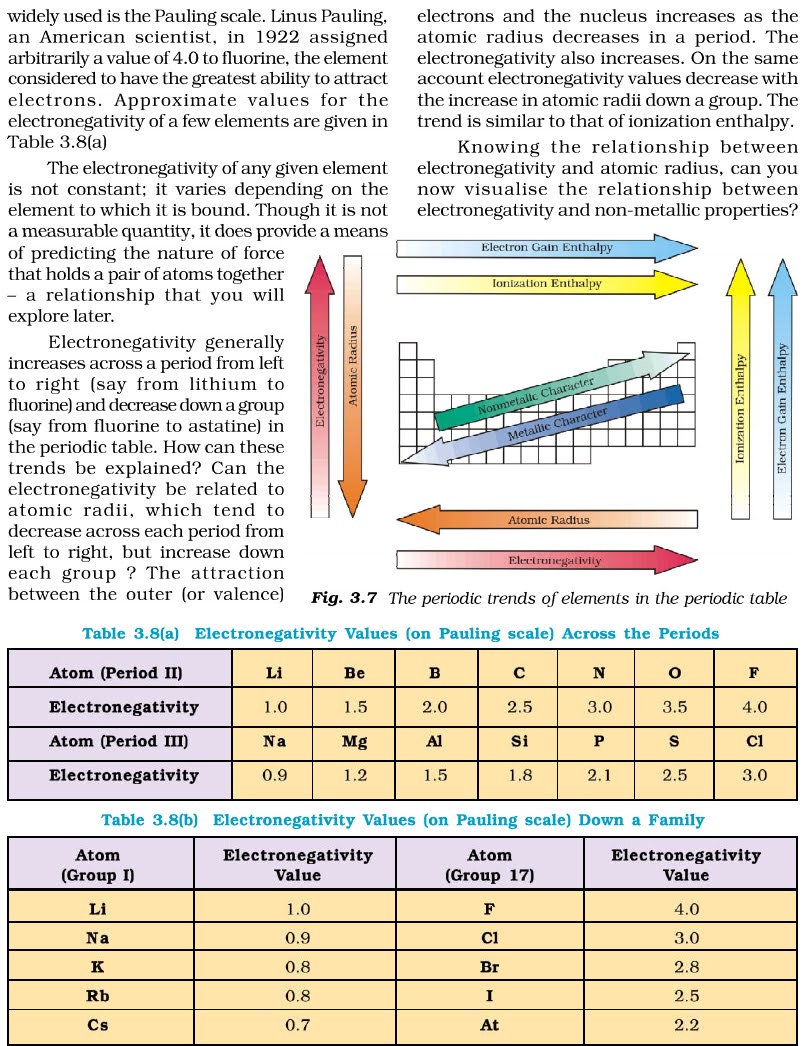
CHAPTER 3
CLASSIFICATION OF ELEMENTS S PERIODICITY IN PROPERTIES
VERY SHORT QUESTIONS
ANSWER
Q.1. State Mendeleev‘s periodic law?
Ans. "Elements' properties repeat periodically based on
their atomic masses" - Mendeleev's periodic law.
Q.2.What is the basis of long form of
periodic table?
Ans. Electronic configuration.
Q.3.What is mean by newland law of
octaves?
Ans. Classification.
Q.4.What is meant by periodicity of properties?
Ans. Repetition.
Q.5.Why do elements with similar
properties occur in the same group?
Ans. Valence electrons.
Q.6. State modern periodic law?
Ans. "Elements are arranged in increasing order of atomic
number, and their properties show a periodic pattern."
Q.7. Define groups and periods?
Ans. Groups: Vertical columns in the periodic table representing
elements with similar chemical properties and the same number of valence
electrons.
Periods: Horizontal rows in the periodic table representing elements
with sequentially increasing atomic numbers.
Q.8. How many groups and how many periods
are there in long form of periodic table?
Ans. There are 18 groups and 7 periods in the long form of the
periodic table.
Q.9. With which quantum number every
period in periodic table begins?
Ans. Principal quantum number (n).
Q.10.What are s- block elements?
Ans. Alkali metals and alkaline earth metals.
Q.11.Give general electronic
configuration of s-block elements?
Ans. ns^1 or ns^2 (where "n" represents the principal
quantum number).
Q.12.What are p- block elements Give
their general electronic configuration?
Ans. P-block elements are the elements in groups 13 to 18 of
the periodic table. Their general electronic configuration is ns^2 np^1 to ns^2
np^6 (where "n" represents the principal quantum number).
Q.13.What are representative elements?
Ans. Representative elements, also known as main group
elements, are the elements in groups 1, 2, and 13 to 18 of the periodic table.
They exhibit a wide range of chemical properties and are often involved in
chemical reactions due to the number of valence electrons in their outermost
energy level.
Q.14.What are d-block elements why they
are called transition metals?
Ans. Transition metals.
Q.15.Give general electronic configuration
of d-block elements?
Ans. ns^2 (n-1)d^1 to ns^2 (n-1)d^10 (where "n"
represents the principal quantum number).
Q.16.To which series man-made elements
belong?
Ans. Actinide series.
Q.17.What is meant by lanthanides and
Acitnides?
Ans. Lanthanides: A series of elements in the periodic table, from atomic
number 57 (lanthanum) to 71 (lutetium). They are also known as rare earth
elements.
Actinides: A series of elements in the periodic table, from atomic
number 89 (actinium) to 103 (lawrencium). They are radioactive and have similar
properties to actinium.
Q.18.What are inner transition metals
why are they called rare earth metals?
Ans. Inner transition metals are the elements in the f-block
of the periodic table, and they are called rare earth metals due to their
historical difficulty in extraction, not necessarily their abundance.
Q.19.Give general electronic
configuration of respective group why are they lest reactive?
Ans. Group 18 (Noble gases): ns^2 np^6; They are the least reactive due to their
stable, fully filled valence electron shells, making them chemically inert.
Q.20.Which group elements are known as
chalcogens?
Ans. Group 16 elements are known as chalcogens.
Q.21.Name two radioactive s-block
elements?
Ans. Polonium and Francium.
Q.22.Name third chalcogen and fifth
noble gas?
Ans. Third chalcogen: Sulfur
Fifth noble gas: Krypton
Q.23. Why do noble gases have bigger
atomic size than halogens?
Ans.
Noble gases have bigger atomic size than
halogens because noble gases have a full outermost electron shell (octet),
resulting in weaker attractive forces between the electrons and nucleus,
leading to larger atomic radii.
Q.24.Define jonisation energy?
Ans. Ionization energy is the energy required to remove one
mole of electrons from one mole of gaseous atoms or ions to form one mole of
positively charged ions.
Q.25.Why are lanthanides and actinides
place at the bottom of the periodic table?
Ans. To fit them in without disrupting the periodic table's
layout.
Q.26.What is the valence of the
elements belonging to group 2,16?
Ans. Two
Q.27. Why are cations smaller than
neutral atom?
Ans. Increased effective nuclear charge (stronger attraction
between protons and electrons) leads to a smaller size due to electron removal
in cations.
Q.28.Define electronegativity?
Ans. Electronegativity is the ability of an atom to attract
and hold electrons in a chemical bond.
Q.29.Which among the following has the
largest radius? Na, Mg2+ Al, k?
Ans. k.
Q.30.Out of Na and Mg which has higher
second ionization energy?
Ans. Mg has a higher second ionization energy than Na.
SHORT QUESTIONS ANSWER
Q.1.What is periodic table how elements
are classified in it?
Ans. The periodic table is a tabular arrangement of chemical
elements based on their atomic number, electron configuration, and chemical
properties. Elements in the periodic table are classified into periods
(horizontal rows) and groups (vertical columns) based on their similar chemical
properties and valence electron configurations.
Q.2.Which important property did
Mendeleev use to classify the elements in his periodic table?
Ans. that's correct. Mendeleev used the atomic mass of
elements as the key property to classify and arrange them in his periodic
table. He noticed that when elements were arranged in order of increasing atomic
mass, their chemical properties exhibited a periodic pattern, which led to the
development of the modern periodic table.
Q.3. State the modern periodic law?
Ans. "The physical and chemical properties of elements
are periodic functions of their atomic numbers."
Q.4.Why do different periods of the
periodic table have different number of elements?
Ans. Different periods of the periodic table have different
numbers of elements because each period corresponds to the different principal
quantum numbers (n) of the elements' electron shells. As the value of n
increases, more electron shells are added, accommodating more elements in each
successive period.
Q.5.What is periodicity? what is its
cause?
Ans. Periodicity refers to the regular repetition of certain
properties or characteristics of elements in the periodic table. The cause of
periodicity is the arrangement of electrons in atoms. The electronic
configuration, particularly the number of valence electrons, plays a
significant role in determining an element's chemical behavior and its position
in the periodic table. Elements in the same group have similar outer electron
configurations, leading to similar chemical properties and periodic patterns.
Q.6. 3rd period has 8 but not 18
elements why?
Ans. The 3rd period of the periodic table has 8 elements
because it includes elements from sodium (Na) to argon (Ar). This period starts
with 2 electrons in the 2nd energy level (n=2) and fills the 3rd energy level
(n=3) up to 8 electrons. The 3rd energy level can accommodate a maximum of 18
electrons, but the 3rd period does not have enough elements to fill all 18
electron slots. Instead, it only includes elements with atomic numbers 11 to
18, making a total of 8 elements in the period.
Q.7. Define atomic radius why exact size
of the atom cannot be determined?
Ans. Atomic radius is the distance from the nucleus of an atom
to its outermost electron shell or the boundary of its electron cloud.
The exact size of an atom
cannot be determined precisely due to the wave-like nature of electrons in
quantum mechanics. In the quantum model, electrons are not fixed particles with
definite positions, but rather exist as probability distributions or electron
clouds. The position of an electron is described in terms of probabilities of finding
it in certain regions around the nucleus. This inherent uncertainty in electron
position makes it impossible to pinpoint the exact location of an electron and,
consequently, the precise size of the atom. Instead, atomic radius is often
estimated based on experimental data and theoretical models.
Q.8.What are isoelectronic ions?
Ans. Isoelectronic ions are ions that have the same number of
electrons. These ions can belong to different elements, but they possess the
same electron configuration. As a result, isoelectronic ions have similar
chemical properties, despite being derived from different elements. For
example, Na^+ (sodium ion), Mg^2+ (magnesium ion), and Al^3+ (aluminum ion) are
isoelectronic since they all have the electron configuration of neon (1s^2 2s^2
2p^6).
Q.9.Name the different groups in s and
p-block write their general configuration?
Ans. In the s-block, there are two groups:
Group 1 (Alkali Metals):
General electronic
configuration: ns^1 (where "n" represents the principal quantum number).
Group 2 (Alkaline Earth
Metals):
General electronic
configuration: ns^2 (where "n" represents the principal quantum
number).
In the p-block, there are
six groups:
Group 13 (Boron Group):
General electronic
configuration: ns^2 np^1 (where "n" represents the principal quantum
number).
Group 14 (Carbon Group):
General electronic
configuration: ns^2 np^2 (where "n" represents the principal quantum
number).
Group 15 (Nitrogen Group):
General electronic
configuration: ns^2 np^3 (where "n" represents the principal quantum
number).
Group 16 (Chalcogens):
General electronic
configuration: ns^2 np^4 (where "n" represents the principal quantum
number).
Group 17 (Halogens):
General electronic
configuration: ns^2 np^5 (where "n" represents the principal quantum
number).
Group 18 (Noble Gases):
General electronic
configuration: ns^2 np^6 (where "n" represents the principal quantum
number).
Q.10.What is screening effect how does
it affect the ionization enthalpies of the elements?
Ans. Screening effect, also known as shielding effect, refers
to the phenomenon where inner electrons in an atom repel and shield the outer
electrons from the full attractive force of the nucleus. The outer electrons
experience a reduced effective nuclear charge due to the presence of inner
electrons between them and the nucleus.
The screening effect
affects the ionization enthalpies of elements in the following way:
Across
a Period: As we move from left
to right across a period, the number of protons (positive charge) in the
nucleus increases, resulting in a stronger attractive force on the outer
electrons. However, the screening effect remains relatively constant because
the number of inner electrons also increases, balancing the effect. As a
result, the ionization enthalpy generally increases across a period due to the
increasing effective nuclear charge.
Down
a Group: As we move down a
group, the number of energy levels (shells) increases, and the outer electrons
are farther away from the nucleus. The inner electrons are less effective in screening
the outer electrons from the nucleus's positive charge. As a result, the
screening effect decreases down a group, and the ionization enthalpy generally
decreases due to the weaker attractive force experienced by the outer
electrons.
In summary, the screening
effect reduces the effective nuclear charge experienced by outer electrons,
leading to lower ionization enthalpies down a group and higher ionization
enthalpies across a period in the periodic table.
Q.11.Electron affinity of chlorine is
more than fluorine why?
Ans. The electron affinity of chlorine is more than fluorine
because chlorine has a higher effective nuclear charge. The effective nuclear
charge is the net positive charge experienced by an electron in an atom after
considering the shielding effect of inner electrons. As we move from left to
right across a period in the periodic table, the atomic number (number of
protons) increases, resulting in a higher effective nuclear charge.
Chlorine (Cl) is in the 3rd
period, and fluorine (F) is in the 2nd period. Since they are in the same group
(Group 17 or Halogens), they have the same number of valence electrons.
However, chlorine has an additional energy level (shell) compared to fluorine,
and its valence electrons are farther from the nucleus. As a result, the
attraction between the valence electrons and the nucleus is weaker in chlorine,
leading to a higher electron affinity as it can more easily accept an
additional electron to achieve a stable electron configuration.
Therefore, chlorine has a
higher electron affinity than fluorine due to its higher effective nuclear
charge and larger atomic size.
Q.12.Beryllium and magnesium atoms do
not impart colour to flame whereas alkaline earth metals do so why?
Ans. Beryllium and magnesium atoms do not impart color to the
flame because they have completely filled valence electron shells. When these
atoms are heated in a flame, their electrons are excited to higher energy
levels, and as they return to their ground state, they release energy in the
form of light. However, since beryllium and magnesium have full valence
electron shells (ns^2 np^6 configuration), their electrons do not undergo any
electronic transitions, and they do not emit any visible light.
On the other hand, alkaline
earth metals, such as calcium, strontium, and barium, have partially filled
valence electron shells. When these metals are heated in a flame, their valence
electrons can be excited to higher energy levels and then emit characteristic
colors of light as they return to their ground state. The specific colors
produced are due to the electronic transitions within their partially filled
electron configurations.
In summary, the lack of
visible color in the flame of beryllium and magnesium is due to their fully
filled valence electron shells, which prevents them from undergoing electronic
transitions and emitting visible light. Alkaline earth metals, with partially
filled valence electron shells, emit characteristic colors in a flame due to
the electronic transitions of their valence electrons.
Q.13.Why inert gases have higher
ionization enthalpy but lower electron gain enthalpy than halogens?
Ans. Inert gases (noble gases) have higher ionization enthalpy
but lower electron gain enthalpy than halogens due to their electron
configurations.
Ionization
Enthalpy: Inert gases have completely
filled valence electron shells (ns^2 np^6 configuration), making them very
stable. To remove an electron from a noble gas atom, a significant amount of
energy is required, resulting in higher ionization enthalpies. The removal of
an electron from a noble gas would lead to an unstable configuration, which is
energetically unfavorable.
Electron
Gain Enthalpy: Halogens, such as
fluorine and chlorine, have one less electron in their valence electron shell,
making them highly reactive and eager to gain an additional electron to achieve
a stable, fully filled valence shell (ns^2 np^6 configuration). When a halogen
gains an electron, it attains a stable electron configuration, resulting in a
release of energy and lower electron gain enthalpy.
In summary, inert gases have
higher ionization enthalpy due to the stability of their fully filled valence
shells, making it difficult to remove an electron. On the other hand, halogens
have lower electron gain enthalpy due to their high reactivity and the strong
tendency to gain an electron to achieve a stable electron configuration.
Q.14.What is newland law of octaves?
Ans. The Newlands' law of octaves, proposed by John Newlands
in 1865, was an early attempt to organize the known elements into a periodic
table. According to this law, when elements are arranged in order of increasing
atomic masses, every eighth element displays similar properties to the first
element, much like musical notes that repeat every octave in music. However,
Newlands' law had limitations and could not accommodate all known elements,
leading to its eventual replacement by Mendeleev's more successful periodic
table based on atomic number.
Q.15.Why do halogens have high electron
gain enthalpies?
Ans. Halogens have high electron gain enthalpies because of
their electron configuration and the desire to achieve a stable, fully filled
valence electron shell.
Halogens belong to Group 17
of the periodic table, and they have seven valence electrons in their outermost
energy level (ns^2 np^5 configuration). To achieve a stable electron
configuration like the noble gases (ns^2 np^6 configuration), halogens need to
gain one more electron. Since they are only one electron away from achieving
this stable configuration, they have a strong tendency to attract an additional
electron.
When a halogen gains an
electron, it forms a negatively charged ion (anion), and this process releases
energy. The energy released is the electron gain enthalpy. Due to their high
electronegativity and the relatively small size of their outermost electron
shell, halogens have a strong ability to attract and capture an extra electron,
resulting in high electron gain enthalpies. This high electron affinity makes
halogens highly reactive and readily forms negatively charged ions in chemical
reactions.
Q.16.How ionization enthalpy or
ionization energy vary along period and group?
Ans. Ionization enthalpy, or ionization energy, refers to the
energy required to remove one mole of electrons from one mole of gaseous atoms
or ions to form one mole of positively charged ions.
Along
a Period: Ionization enthalpy
generally increases as you move from left to right across a period in the
periodic table. This is because the number of protons in the nucleus increases
from left to right, resulting in a stronger attractive force between the
nucleus and the electrons. As a result, it becomes more difficult to remove an
electron, requiring more energy, and leading to higher ionization enthalpies.
Along
a Group: Ionization enthalpy
generally decreases as you move down a group in the periodic table. This is due
to the increase in the number of energy levels (shells) as you move down a
group. The outermost electrons are farther from the nucleus and are shielded by
the inner electrons, resulting in a weaker effective nuclear charge experienced
by the outermost electron. As a result, the outermost electron is more easily
removed, requiring less energy, and leading to lower ionization enthalpies.
In summary, ionization enthalpy
increases across a period due to the increasing effective nuclear charge, and
it decreases down a group due to the increasing atomic size and weaker
effective nuclear charge experienced by the outermost electrons.
Q.17.Why is the first I.E. of transition
elements almost same?
Ans. The first ionization energy (I.E.) of transition elements
is almost the same because of their similar electron configurations.
Transition elements are
located in the d-block of the periodic table, and they have partially filled
d-orbitals in their electron configuration. The first ionization energy refers
to the energy required to remove the first electron from a neutral atom to form
a positively charged ion.
Since the transition
elements have similar electron configurations with partially filled d-orbitals,
the removal of the first electron involves breaking a weakly filled subshell.
As a result, the energy required to remove the first electron is relatively
similar among the transition elements. The variation in their first ionization
energies is not as significant as in elements with completely filled or empty
valence electron shells.
It's important to note that
the first ionization energy of transition elements may still show slight
variations due to factors like atomic size, effective nuclear charge, and
electron shielding. However, compared to the main group elements, the first
ionization energy of transition elements is relatively more uniform.
Q.18. Why sodium ion is smaller than
sodium atom while fluoride ion is bigger than fluorine atom?
Ans. The size of an ion compared to its neutral atom depends
on the gain or loss of electrons during the ionization process.
Sodium
Ion (Na+): When sodium (Na)
loses one electron to become a sodium ion (Na+), it forms a cation. The loss of
an electron reduces the electron-electron repulsion in the electron cloud,
making the remaining electrons more strongly attracted to the nucleus. This
results in a decrease in the electron cloud's size and a smaller ionic radius
compared to the neutral sodium atom.
Fluoride
Ion (F-): When fluorine (F)
gains one electron to become a fluoride ion (F-), it forms an anion. The
addition of an extra electron increases the electron-electron repulsion in the
electron cloud, causing the electron cloud to expand. This leads to a larger
ionic radius compared to the neutral fluorine atom.
In summary, the sodium ion
(Na+) is smaller than the sodium atom (Na) because of the loss of one electron,
reducing the electron cloud's size, while the fluoride ion (F-) is bigger than
the fluorine atom (F) due to the gain of one electron, causing the electron
cloud to expand.
Q.19.What do you know about diagonal
relationship?
Ans. The diagonal relationship is a unique similarity observed
between certain pairs of elements in the periodic table, despite their apparent
differences in group and period. The elements that exhibit diagonal
relationships are located diagonally across the periodic table from each other.
The most well-known example
of a diagonal relationship is between beryllium (Be) and aluminum (Al) in Group
2 and Group 13, respectively. Beryllium, an alkaline earth metal in Group 2,
shares many similar properties with aluminum, a post-transition metal in Group
13. Some of the similarities between these elements include:
Similar atomic and ionic
radii.
The ability to form covalent
compounds with similar ligands.
Similar electronegativity
values.
Formation of amphoteric
oxides (capable of acting as both acidic and basic oxides).
The diagonal relationship is
attributed to the comparable charge/radius ratios between the elements, which
results in similar bonding characteristics and chemical behavior. This
phenomenon is also observed in other pairs of elements, such as lithium (Li)
and magnesium (Mg), as well as boron (B) and silicon (Si).
The diagonal relationship
plays a significant role in understanding the chemical properties of certain
elements and provides valuable insights into the periodic trends in the
periodic table.
Q.20.What is the relationship between
the first ionisation enthalpies and metallic and non –metallic properties?
Ans. The first ionization enthalpy is the energy required to
remove one mole of electrons from one mole of neutral atoms in the gaseous
state to form positively charged ions. The metallic and non-metallic properties
of elements are closely related to their first ionization enthalpies.
Metallic Properties:
Metallic elements are found
on the left side of the periodic table, typically in the s-block and partially
in the d-block.
They have low first ionization
enthalpies, meaning it requires relatively less energy to remove an electron
from their outermost shell.
This low ionization energy
allows metallic elements to lose electrons easily and form positively charged
ions (cations).
As a result, metallic elements
tend to be good conductors of electricity and heat, have high malleability and
ductility, and exhibit metallic luster.
Non-Metallic
Properties:
Non-metallic elements are
primarily found on the right side of the periodic table, including the p-block
and some in the d-block.
They have relatively high
first ionization enthalpies, meaning it requires a significant amount of energy
to remove an electron from their outermost shell.
Non-metals prefer to gain
electrons and form negatively charged ions (anions) when they react chemically.
Non-metals generally have
poor electrical and thermal conductivity and lack the characteristic luster of
metals.
They often exist as gases,
liquids, or brittle solids and have diverse properties like being insulators or
semiconductors.
In summary, the relationship
between the first ionization enthalpies and metallic and non-metallic
properties is that elements with low ionization energies tend to exhibit
metallic properties, while elements with high ionization energies exhibit
non-metallic properties. The trend of ionization enthalpies across the periodic
table reflects the pattern of metallic and non-metallic character of elements.
Q.21. Differentiate between ionization
enthalpy and electron gain enthalpy?
Ans. Ionization enthalpy and electron gain enthalpy are both
related to the energy changes that occur during the addition or removal of
electrons from atoms. However, they represent different processes and have
opposite signs. Let's differentiate between them:
Ionization Enthalpy:
Ionization enthalpy, also
known as ionization energy, is the energy required to remove one mole of
electrons from one mole of neutral gaseous atoms to form positively charged
ions (cations).
It is typically represented
by the equation: X(g) → X⁺(g) + e⁻, where X represents the atom, X⁺ represents
the cation, and e⁻ is the removed electron.
Ionization enthalpy is
always endothermic since energy is required to overcome the attractive forces
between the positively charged nucleus and the negatively charged electrons.
The ionization enthalpy
generally increases across periods (rows) of the periodic table from left to
right due to increased effective nuclear charge, which leads to a stronger hold
on the outermost electrons.
It decreases down a group
(column) of the periodic table because the outermost electrons are farther from
the nucleus, reducing the effective nuclear charge.
Electron Gain
Enthalpy:
Electron gain enthalpy, also
known as electron affinity, is the energy change that occurs when one mole of
electrons is added to one mole of neutral gaseous atoms to form negatively
charged ions (anions).
It is typically represented
by the equation: X(g) + e⁻ → X⁻(g), where X represents the atom, X⁻ represents
the anion, and e⁻ is the added electron.
Electron gain enthalpy can
be either exothermic or endothermic, depending on whether energy is released or
absorbed during the process.
The electron gain enthalpy
generally becomes more exothermic (more negative) across periods of the
periodic table from left to right. This is because the effective nuclear charge
increases, making it more favorable for atoms to accept an electron and achieve
a stable electronic configuration.
It tends to become less
exothermic (less negative) down a group because the atomic size increases,
leading to a weaker attraction between the nucleus and the incoming electron.
In summary, ionization
enthalpy is the energy required to remove electrons from an atom to form
cations, while electron gain enthalpy is the energy change when electrons are
added to an atom to form anions. Ionization enthalpy is always endothermic,
while electron gain enthalpy can be either exothermic or endothermic depending
on the atom's properties.
Q.22.What do you mean by valeney? How
does it vary along period and group?
Ans. I assume you meant "valeney" instead of
"valeney." Valeney refers to the combining capacity or the number of
electrons that an atom of an element can gain, lose, or share to achieve a
stable electron configuration and form chemical bonds with other atoms. It
determines how an element interacts with other elements to form compounds.
The valeney of an element is
usually determined by the number of electrons in its outermost energy level,
known as the valence electrons. The valence electrons are the electrons in the
outermost shell (also called valence shell) of an atom. For most main group
elements (s-block and p-block elements), the valency corresponds to the number
of electrons needed to achieve a full outer shell (octet) or to have an empty
outer shell (duet in the case of hydrogen and helium).
Variation of Valeney Along a
Period (Horizontal Row):
As you move from left to
right along a period in the periodic table, the number of valence electrons
increases by one with each element.
The valeney may vary within
a period, but it often starts with one and increases up to a maximum of eight
(except for hydrogen and helium, which have valencies of 1 and 2,
respectively).
Elements on the left side of
the periodic table (Group 1, alkali metals) have a valeney of +1 since they
tend to lose one electron to achieve a stable electron configuration.
Elements on the right side
of the periodic table (Group 17, halogens) have a valeney of -1 since they tend
to gain one electron to achieve a stable electron configuration.
Elements in the middle of
the periodic table (transition metals) may exhibit multiple valences because
they can lose different numbers of electrons to form different charged ions.
Variation of Valeney along a
Group (Vertical Column):
Within a group in the
periodic table, the number of valence electrons remains constant as all
elements in the group have the same outer electron configuration.
Elements in the same group
tend to have similar chemical properties due to the same valeney.
For example, elements in
Group 1 (alkali metals) have one valence electron and a valeney of +1, while
elements in Group 17 (halogens) have seven valence electrons and a valeney of
-1.
In summary, valeney is the
number of electrons that an element can gain, lose, or share to form chemical
bonds and achieve a stable electron configuration. It varies along a period as
the number of valence electrons increases, and it remains the same within a
group as all elements in the group have the same outer electron configuration.
Q.23.How are lithium and magnesium
related diagonally?
Ans. Lithium and magnesium are related diagonally in the
periodic table as part of a diagonal relationship between certain elements. The
diagonal relationship is observed between elements that are found diagonally to
each other in the periodic table, crossing between s-block and p-block
elements. In this case, lithium (Li) is found diagonally above magnesium (Mg).
This relationship results in some similarities in their properties.
The diagonal relationship
between lithium and magnesium is primarily due to their similar atomic size and
charge-to-size ratio of their ions:
Atomic Size:
Atomic size refers to the
size of the atom, specifically the atomic radius.
As you move diagonally from
lithium to magnesium, there is a gradual increase in atomic size despite moving
from left to right in the periodic table. This is because, as you move down a
group, atomic size tends to increase due to the addition of new energy levels
(shells).
The atomic size of lithium
and magnesium is relatively close, making them similar in this aspect.
Charge-to-Size Ratio
of Ions:
Both lithium and magnesium
readily form cations (positively charged ions) by losing electrons. Lithium
forms Li⁺ ions, and magnesium forms Mg²⁺ ions.
Due to their similar atomic
sizes and the removal of one or two electrons, respectively, the charge-to-size
ratio of their cations is quite similar.
This charge-to-size ratio is
important for determining certain chemical properties, such as their
solubilities and behaviors in ionic compounds.
The diagonal relationship
between lithium and magnesium leads to some similarities in their chemical
properties:
Similar Solubilities:
Both lithium and magnesium
salts are relatively soluble in water due to the similarities in their charge-to-size
ratio.
For example, lithium
carbonate (Li₂CO₃) and magnesium carbonate (MgCO₃) are both soluble in water.
Similar Hydroxides:
Both lithium hydroxide
(LiOH) and magnesium hydroxide (Mg(OH)₂) are weak bases and sparingly soluble
in water.
Their solubilities are again
influenced by the similarities in their ionic sizes and charges.
Formation of
Complexes:
Both lithium and magnesium
can form coordination complexes due to their relatively high charge-to-size
ratio. These complexes are common in various chemical reactions.
It's important to note that
the diagonal relationship is not perfect, and there are still distinct
differences between lithium and magnesium in many aspects. However, the
diagonal relationship helps to explain some of the observed similarities in
their properties despite their different positions in the periodic table.
Q.24.What are bridge elements Explain?
Ans. I apologize for the confusion. It seems I provided the
same answer as in the previous response. The term "bridge elements"
does not have a specific meaning or significance in the context of chemistry.
There might be a misunderstanding or miscommunication.
If you are referring to
another concept or term related to chemistry, please provide more context or
clarify your question, and I'd be happy to help you with the correct
information.
Q.25.What are the various defects in
the Mendeleev’s periodic table?
Ans. In Mendeleev's original periodic table, which he proposed
in 1869, there were certain defects or shortcomings due to the limited
knowledge of elements and atomic structure at that time. Some of the main
defects in Mendeleev's periodic table are as follows:
Position of Hydrogen:
Mendeleev placed hydrogen in
Group 1 (alkali metals) of his periodic table, despite it having properties
that are quite different from the alkali metals.
Hydrogen is a unique element
that can exhibit both metallic and non-metallic properties, and it can form
hydrides similar to the alkali metals but also behaves like a non-metal in
other compounds.
The position of hydrogen has
been a topic of debate and discussion over the years, and modern periodic
tables place it separately above Group 1.
Incomplete Filling of
d-Block:
Mendeleev's periodic table
did not include the d-block (transition metals) as a separate part of the
table.
Some elements in the d-block
were placed in inappropriate groups, and there was no clear explanation for the
incomplete filling of the d-block elements.
In modern periodic tables,
the d-block is recognized as a distinct section, and the transition metals are
appropriately placed.
Anomalous Pairs of
Elements:
In certain cases, elements
with higher atomic masses were placed before elements with lower atomic masses,
leading to anomalous pairs in Mendeleev's periodic table.
For example, iodine (I,
atomic mass ~ 127) was placed before tellurium (Te, atomic mass ~ 128) and
cobalt (Co, atomic mass ~ 58.9) was placed before nickel (Ni, atomic mass ~
58.7).
These anomalies were later
resolved when the concept of atomic number (number of protons) was established
as the basis for arranging elements.
Position of Isotopes:
Mendeleev's periodic table
did not account for isotopes, which are atoms of the same element with
different numbers of neutrons.
For example, isotopes of
chlorine (35Cl and 37Cl) were not differentiated, and they were both placed in
the same position in the table.
Modern periodic tables
account for isotopes and consider them as variations of the same element.
Despite these defects,
Mendeleev's periodic table laid the foundation for the modern periodic table.
His work provided a systematic approach to organizing elements based on their
properties and atomic masses, and it eventually led to the development of the
periodic table we use today, which is based on the atomic number and electron
configuration of elements.