CHAPTER 4 CHEMICAL BONDING AND MOLECULAR STRUCTURE
VERY SHORT QUESTIONS
ANSWER
Q.1. Define a chemical bond?
Ans. A chemical bond is a force of attraction that holds atoms
together in a molecule or compound.
Q.2.What is modern concept of chemical
bonding?
Ans. Atoms bond by sharing, gaining, or losing electrons to
achieve a stable electron configuration and form molecules or compounds.
Q.3.Is ionic bond directional?
Ans. No, ionic bonds are not directional.
Q.4.Which factors are responsible for
the formation of ionic bond?
Ans. The main factors responsible for the formation of an
ionic bond are the large difference in electronegativity between the atoms
involved and the transfer of electrons from one atom to another.
Q.5. Define a covalent bond?
Ans. A covalent bond is a chemical bond formed by the sharing
of electrons between two atoms to achieve a stable electron configuration.
Q.6. Distinguish between electrovalency
and covalency?
Ana. Electrovalency involves the complete transfer of
electrons, resulting in the formation of ions, while covalency involves the
sharing of electrons between atoms.
Q.7.Deifne electrovalency of an
element?
Ans. Electrovalency of an element refers to the number of
electrons that an atom gains or loses to form an ion during the formation of an
ionic bond.
Q.8.What is sigma bond?
Ans. A sigma bond is a type of covalent bond formed by the
direct overlap of atomic orbitals along the bond axis.
Q.9.Why is solid nacl not conductor of
electricity?
Ans. Solid NaCl (table salt) is not a conductor of electricity
because it is a non-metallic ionic compound with fixed ions in a crystal
lattice, and there are no free-moving electrons or ions to carry electric
charge.
Q.10. Define covalency of an element?
Ans. Covalency of an element refers to the number of covalent
bonds that an atom can form with other atoms to achieve a stable electron
configuration.
Q.11.Define bond order?
Ans. Bond order is the number of electron pairs shared between
two atoms in a covalent bond, representing the strength of the bond.
Q.12.Define electronegativity?
Ana. Electronegativity is the ability of an atom to attract
electrons towards itself in a chemical bond.
Q.13.Define hybridization?
Ans. Hybridization is the mixing of atomic orbitals to form
new hybrid orbitals with different shapes and energies for bonding in
molecules.
Q.14. Define metallic bond?
Ans. Metallic bond is the electrostatic attraction between
positive metal ions and delocalized electrons, resulting in a cohesive force
that holds metal atoms together in a lattice structure.
Q.15.Why is copper more malleable and
ductile than brass?
Ans. Crystal structure and bonding in copper provide higher
malleability and ductility compared to the alloy brass.
Q.16. Why do transition metals have high
melting point?
Ans. Strong metallic bonding.
Q.17.Why are metals good conductor of
heat and electricity in solid state?
Ans. Delocalized electrons.
Q.18. Define dipole moment Is it scalar
or vector quantity?
Ans. Dipole moment is a measure of the polarity of a covalent
bond and is a vector quantity.
Q.19. Define Debye?
Ans. Debye is a unit used to measure the dipole moment in a
molecule. It is equal to 3.336 x 10^-30 coulomb-meter.
Q.20.Define H- bond?
Ans. Hydrogen bond is a strong type of non-covalent bond
formed between a hydrogen atom and a highly electronegative atom (e.g., oxygen,
nitrogen, or fluorine) in a different molecule or within the same molecule.
Q.21.Define resonance?
Ans. Resonance is a phenomenon in which multiple Lewis
structures with different arrangements of electrons contribute to the true
structure of a molecule or ion.
Q.22.What type of atomic orbitals
overlap of form molecular orbitals?
Ans. Atomic orbitals of the same type (e.g., s-s, p-p, d-d)
overlap to form molecular orbitals.
Q.23. How is bond length related to
stability of a molecule?
Ans. Shorter bond length generally indicates higher bond
strength and greater stability in a molecule.
Q.24. When is a molecule paramagnetic
in nature?
Ans. A molecule is paramagnetic when it has unpaired electrons
in its molecular orbitals.
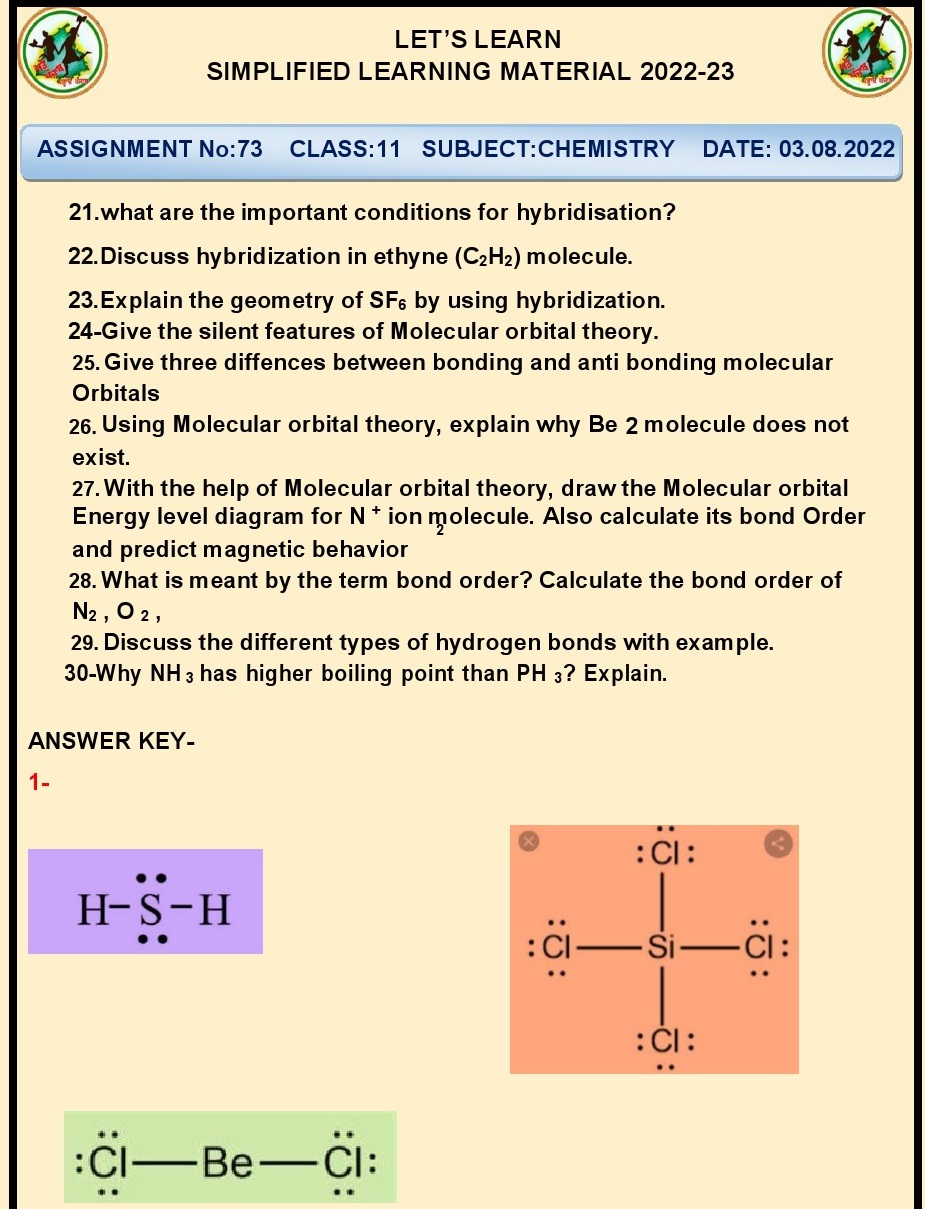
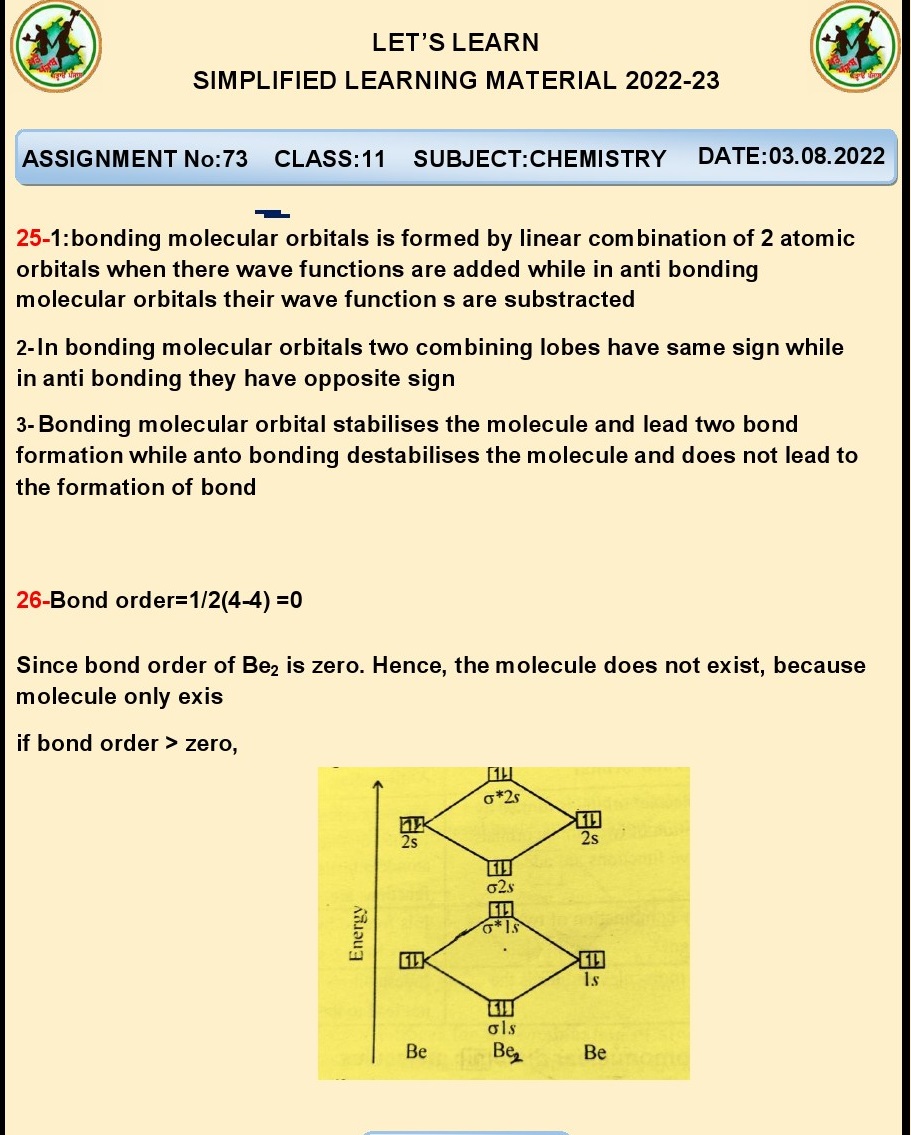
Q.25.Does Be2 molecule exist
justify?
Ans. No, the Be2 molecule does not exist due to its high
energy and instability caused by a lack of a sufficient number of electrons to
form stable bonds.
Q.26.Why do cotton clothes take a long
time do dry as compared to synthetic clothes Give reason?
Ans. Evaporation.
Q.27. Benzene is a stable molecule
inspite of the fact that it consists of three double bonds why?
Ans. Resonance.
Q.28. Write the electronic
configuration of B2.
Ans. B2: (σ 2 s) 2 (σ 2 s) 2
SHORT QUESTIONS ANSWER
Q.1. Explain the cause of covalent bond
for formation?
Ans. The cause of the covalent bond formation is the sharing
of electrons between two atoms to achieve a stable electron configuration and
lower the overall energy of the system. This sharing of electrons allows both
atoms to fill their valence shells and attain a more stable electronic
arrangement similar to noble gases. Covalent bonds typically form between
non-metal atoms with similar electronegativities, allowing them to share
electrons and become more stable.
Q.2.Give conditions to form covalent
bond according to valence bond theory?
Ans. According to the valence bond theory, covalent bonds form
under the following conditions:
Overlapping
Atomic Orbitals: Covalent bonds are
formed when the atomic orbitals of two atoms overlap with each other.
Compatible
Orbitals: The overlapping
orbitals should have similar energy levels and compatible shapes for effective
sharing of electrons.
Proper
Orientation: The overlapping
orbitals should have the correct spatial orientation to ensure efficient
electron sharing.
Unpaired
Electrons: Covalent bonds occur
when both atoms have unpaired electrons available for sharing in their valence
shells.
Similar
Electronegativity: Covalent
bonds generally form between atoms with similar electronegativities to ensure
equal sharing of electrons.
Specific
Molecular Geometry: The
formation of covalent bonds leads to the creation of specific molecular geometries
based on the arrangement of overlapping orbitals.
Q.3. Define resonance energy what is
the resonance energy of benzene?
Ans. Resonance energy is the stability gained by a molecule or
ion through resonance, which arises from the delocalization of electrons over
multiple resonance structures.
The resonance energy of
benzene is approximately 150 kJ/mol. This means that the actual energy of
benzene is lower (more stable) than any of its individual resonance structures
by about 150 kJ/mol due to the delocalization of π electrons over the six
carbon atoms in the benzene ring.
Q.4. Give conditions for resonating
structures?
Ans. The conditions for the formation of resonating structures
are as follows:
Delocalized
Electrons: Resonating structures
involve molecules or ions with delocalized electrons, typically represented by
pi (π) bonds or lone pairs.
Multiple
Bonds: Resonance occurs when
there are multiple bonds (double or triple bonds) between atoms.
Similar
Connectivity: The connectivity of
the atoms remains the same in all resonating structures, with only the distribution
of electrons varying.
Valid
Lewis Structures: Each
resonating structure must obey the octet rule and have the correct number of valence
electrons for each atom.
Similar
Energy Levels: Resonating structures
should have comparable energy levels to contribute significantly to the overall
stability of the molecule or ion.
Minimized
Charge Separation: Resonating
structures are preferred when they minimize charge separation and maintain a more
even distribution of charge.
Overall, resonating
structures represent different possible arrangements of electrons in a molecule
or ion that contribute to its overall stability through delocalization and
electron distribution.
Q.5.Give four differences between ionic
and covalent compounds?
Ans. Four differences between ionic and covalent compounds
are:
Bonding Type:
Ionic compounds are formed
through the transfer of electrons from one atom to another, resulting in the
formation of oppositely charged ions that are held together by electrostatic
forces.
Covalent compounds are
formed through the sharing of electrons between atoms to achieve a stable
electron configuration.
Nature of Bond:
Ionic bonds are
electrostatic attractions between positively charged cations and negatively
charged anions.
Covalent bonds are formed by
the overlapping of atomic orbitals and involve the sharing of electrons between
atoms.
State at Room
Temperature:
Ionic compounds are often
solids at room temperature, characterized by high melting and boiling points,
and they conduct electricity when dissolved in water or molten due to the
presence of free ions.
Covalent compounds can be
found as gases, liquids, or solids at room temperature, depending on the
molecule's size and intermolecular forces. Most covalent compounds do not
conduct electricity in any state.
Solubility in Water:
Ionic compounds are
generally soluble in water due to the attraction between ions and water
molecules, which can surround and dissolve the ions in the solution.
Covalent compounds vary in
their solubility in water. Some covalent compounds can dissolve in water if
they have polar covalent bonds and interact favorably with water molecules,
while non-polar covalent compounds tend to be insoluble in water.
Q.6. Why is HI stronger acid than HF?
Ans. Hydrogen iodide (HI) is a stronger acid than hydrogen
fluoride (HF) because the iodine atom is larger than the fluorine atom, leading
to weaker bond strength between hydrogen and iodine compared to hydrogen and
fluorine. As a result, the hydrogen ion (H⁺) is more easily released from HI,
making it a stronger acid in aqueous solutions. Additionally, the larger size
of iodine allows for better stabilization of the negative charge on the iodide
ion (I⁻) after the acid dissociates, further enhancing the acidic strength of
HI.
Q.7.Why is HF liquid whereas HCI gas?
Ans. Hydrogen fluoride (HF) is a liquid at room temperature
and atmospheric pressure, while hydrogen chloride (HCl) is a gas at the same
conditions due to differences in their intermolecular forces.
HF has stronger
intermolecular forces known as hydrogen bonding, which requires more energy to
break the attractions between molecules. These hydrogen bonds hold the HF
molecules together, resulting in a liquid state at room temperature. On the
other hand, HCl molecules experience weaker van der Waals forces, which are
easier to overcome, leading to a gas phase at room temperature and atmospheric
pressure.
Q.8. State octet rule Give two
exainples following octet rule?
Ans. Octet Rule: Atoms tend to gain, lose, or share electrons to achieve a
stable electron configuration with eight valence electrons (except for hydrogen
and helium, which achieve stability with two valence electrons).
Examples:
Sodium
Chloride (NaCl): Sodium
(Na) donates one electron to chlorine (Cl) to achieve a stable electron
configuration, resulting in the formation of Na⁺ and Cl⁻ ions that attract each
other in an ionic bond to form NaCl.
Methane
(CH₄): Carbon (C) shares its
four valence electrons with four hydrogen (H) atoms to achieve a stable octet,
forming four covalent bonds and a stable methane molecule.
Q.9.What are valence electrons?
Ans. Valence electrons are the electrons in the outermost
energy level (valence shell) of an atom. These electrons are involved in
chemical bonding and determine the reactivity and chemical properties of an
element. The number of valence electrons typically corresponds to the group
number of the element in the periodic table for main group elements (s-block
and p-block). For example, elements in Group 1 have one valence electron,
elements in Group 2 have two valence electrons, and so on. Valence electrons
play a crucial role in the formation of chemical bonds and the stability of
atoms and molecules.
Q.10. Define valency?
Ans. Valency is the combining capacity or the number of
electrons that an atom of an element can gain, lose, or share to achieve a
stable electron configuration and form chemical bonds with other atoms. It
determines how an element interacts with other elements to form compounds. The
valency of an element is usually determined by the number of electrons in its
outermost energy level, known as the valence electrons. For main group elements
(s-block and p-block), the valency often corresponds to the number of valence
electrons. For example, elements in Group 1 have a valency of +1, as they tend
to lose one electron to achieve a stable configuration, while elements in Group
17 have a valency of -1, as they tend to gain one electron to achieve
stability. However, for transition metals (d-block), the valency can vary due
to the presence of electrons in both the outermost and inner d orbitals.
Q.11.Name the various types of bonds?
Ans. The various types of bonds are:
Ionic
Bonds: Formed by the
transfer of electrons between atoms, resulting in the attraction between
oppositely charged ions.
Covalent
Bonds: Formed by the sharing
of electrons between atoms to achieve a stable electron configuration.
Metallic
Bonds: Found in metals,
where positive metal ions are held together in a lattice structure by a "sea"
of delocalized electrons.
Hydrogen
Bonds: A strong type of
dipole-dipole interaction between a hydrogen atom and a highly electronegative
atom (e.g., oxygen, nitrogen, or fluorine) in a different molecule or within
the same molecule.
Van
der Waals Forces: Weak
forces between molecules, including London dispersion forces (arising from
temporary fluctuations in electron distribution) and dipole-dipole interactions
(between polar molecules).
Coordinate
Covalent Bonds: A
special type of covalent bond where one atom donates a pair of electrons to
form a bond with another atom or molecule.
Pi
Bonds: A type of covalent
bond formed by the side-to-side overlap of p-orbitals. Pi bonds are present in
double and triple bonds in addition to sigma bonds.
Q.12.What is stoichiometric formulae of
ionic compounds?
Ans. The stoichiometric formula of an ionic compound
represents the ratio of ions present in the compound and is based on the law of
definite proportions. It is the simplest whole-number ratio of cations and
anions that results in a neutral compound.
For example:
Sodium
Chloride (NaCl): The
stoichiometric formula is NaCl, representing one sodium ion (Na⁺) and one
chloride ion (Cl⁻) combined in a 1:1 ratio.
Calcium
Oxide (CaO): The stoichiometric
formula is CaO, representing one calcium ion (Ca²⁺) and one oxygen ion (O²⁻)
combined in a 1:1 ratio.
Magnesium
Nitrate (Mg(NO₃)₂): The
stoichiometric formula is Mg(NO₃)₂, representing one magnesium ion (Mg²⁺) and
two nitrate ions (NO₃⁻) combined in a 1:2 ratio.
The stoichiometric formula
provides important information about the relative proportions of ions present
in an ionic compound, allowing for precise and consistent representation of
chemical compounds.
Q.13.How does electronegativity help to
predict the polarity of the bond?
Ans. Electronegativity is a measure of an atom's ability to
attract electrons towards itself in a chemical bond. The difference in
electronegativity between two atoms in a bond plays a crucial role in
determining the polarity of the bond. The greater the difference in
electronegativity, the more polar the bond becomes.
When two atoms with
significantly different electronegativities form a bond:
If the electronegativity
difference is very small or zero, the bond is non-polar. In a non-polar
covalent bond, the electrons are shared equally between the atoms, resulting in
a balanced distribution of charge.
If the electronegativity
difference is moderate, the bond is polar covalent. In a polar covalent bond,
the more electronegative atom attracts the shared electrons closer to itself,
creating a partial negative charge (δ-) on that atom and a partial positive
charge (δ+) on the other atom.
If the electronegativity
difference is large, the bond becomes ionic. In an ionic bond, the more
electronegative atom effectively gains the shared electrons, forming a negative
ion (anion), while the less electronegative atom loses electrons, forming a
positive ion (cation). The resulting ions are held together by electrostatic
attractions, and the bond is no longer covalent.
Electronegativity helps
predict the polarity of a bond by assessing how the electrons are distributed
between the bonded atoms. It allows us to understand whether the bond is
non-polar, polar covalent, or ionic, which influences the overall properties
and behavior of molecules and compounds.
Q.14.The dipole moment of hydrogen
halide decreases from HF to HI Explain this trend?
Ans. The trend of decreasing dipole moment from HF to HI in
hydrogen halides can be explained by two main factors: the increase in atomic
size and the decrease in electronegativity as we move down the halogen group.
Increase
in Atomic Size: As
we move down the halogen group (from fluorine to iodine), the atomic size of
the halogen atoms increases. Larger atoms have more diffuse electron clouds,
leading to weaker interactions between the shared electrons in the bond and the
positively charged nuclei. This results in a decrease in the magnitude of the
dipole moment.
Decrease
in Electronegativity: Electronegativity
is the ability of an atom to attract electrons towards itself in a chemical bond.
As we move down the halogen group, the electronegativity of the halogens
decreases. Fluorine is the most electronegative element, and its
electronegativity decreases as we move towards iodine. In HF, the high
electronegativity of fluorine causes it to strongly attract the shared
electrons towards itself, resulting in a larger dipole moment. In HI, iodine's
lower electronegativity results in weaker electron-attracting ability, leading
to a smaller dipole moment.
In summary, the decreasing
dipole moment from HF to HI in hydrogen halides is mainly due to the larger
atomic size and the lower electronegativity of iodine compared to fluorine.
These factors weaken the bond polarity and reduce the overall dipole moment in
HI as compared to HF.
Q.15. List out molecules with zero
dipole moment?
Ans. Molecules with zero dipole moment are typically symmetric
in shape and have no net dipole due to the cancellation of dipole moments from
individual bonds. Here are some examples of molecules with zero dipole moment:
Carbon
Dioxide (CO₂): It has a linear
structure, and the two polar C=O bonds have equal and opposite dipole moments,
resulting in a net dipole moment of zero.
Methane
(CH₄): It has a tetrahedral
geometry with four identical C-H bonds arranged symmetrically around the carbon
atom, leading to a net dipole moment of zero.
Ethylene
(C₂H₄): It has a planar
structure with two C-C sigma bonds and two C-H sigma bonds. The dipole moments
of the two C-H bonds cancel out each other, resulting in a net dipole moment of
zero.
Benzene
(C₆H₆): It has a hexagonal
planar structure with alternating single and double bonds. The individual bond
dipole moments cancel each other due to the symmetrical arrangement of carbon
and hydrogen atoms, resulting in a net dipole moment of zero.
Tetrachloromethane
(CCl₄): It has a tetrahedral
geometry with four C-Cl bonds arranged symmetrically around the carbon atom,
leading to a net dipole moment of zero.
These molecules have zero
dipole moment due to their symmetric structures and the equal and opposite
cancellation of individual bond dipole moments.
Q.16.Which out of the two molecules OCS
and CS2 has a higher dipole moment and why?
Ans. The molecule CS₂ (Carbon disulfide) has a higher dipole
moment compared to OCS (Carbonyl sulfide).
In OCS, the molecule has a
linear structure with the oxygen atom between the carbon and sulfur atoms. The
oxygen atom is more electronegative than both carbon and sulfur, creating a
dipole moment between the C-O and S-O bonds. However, the dipole moments of the
two bonds are equal and opposite in direction, resulting in their cancellation.
As a result, the net dipole moment of OCS is close to zero.
In CS₂, the molecule also
has a linear structure with the carbon atom in the middle of two sulfur atoms.
Both carbon-sulfur bonds have a dipole moment in the same direction due to the
difference in electronegativity between carbon and sulfur. As a result, the
dipole moments of the two bonds do not cancel out, leading to a net dipole
moment that gives CS₂ a non-zero dipole moment.
Therefore, CS₂ has a higher
dipole moment compared to OCS.
Q.17.What is a coordinate or dative
bond Explain with help of an example?
Ans. A coordinate bond, also known as a dative bond, is a type
of covalent bond in which both electrons are contributed by one atom. In this
bond, one atom donates a pair of electrons (the electron pair donor) to another
atom that acts as an electron pair acceptor. The electron pair donor is usually
an atom with a lone pair of electrons, and the electron pair acceptor typically
has an electron-deficient orbital.
An example of a coordinate
bond is the formation of the ammonium ion (NH₄⁺) from ammonia (NH₃) and a
hydrogen ion (H⁺):
Ammonia (NH₃) is the
electron pair donor as it has a lone pair of electrons on the nitrogen atom.
The hydrogen ion (H⁺) is the
electron pair acceptor as it has an empty orbital (no electrons).
When ammonia reacts with the
hydrogen ion (H⁺), the lone pair of electrons from the nitrogen atom in ammonia
is donated to the empty orbital of the hydrogen ion, forming a coordinate bond.
The resulting complex is the ammonium ion (NH₄⁺):
NH₃ + H⁺ → NH₄⁺
In the ammonium ion, the
nitrogen atom shares its lone pair of electrons with the hydrogen ion,
resulting in the formation of the coordinate bond between nitrogen and
hydrogen. The ammonium ion has a tetrahedral geometry, with four hydrogen atoms
surrounding the nitrogen atom.
Coordinate bonds play a
crucial role in the formation of coordination complexes, where metal ions act
as electron pair acceptors, and ligands (molecules or ions with lone pairs) act
as electron pair donors. These complexes are vital in various chemical
reactions and are often involved in catalysis and biological processes.
Q.18. Define lattice enthalpy how is it
related to the stability of an ionic compound?
Ans. Lattice enthalpy is the energy released or absorbed when
gaseous ions come together to form a solid ionic lattice. It is a measure of
the strength of the electrostatic forces between the oppositely charged ions in
the lattice. Lattice enthalpy is typically expressed as a negative value, as
energy is released during the formation of the lattice.
The lattice enthalpy is
directly related to the stability of an ionic compound. A higher magnitude of
lattice enthalpy indicates stronger forces of attraction between the ions in
the lattice, leading to a more stable ionic compound. The stronger the
electrostatic interactions between the ions, the more energy is required to
break these interactions, making the ionic compound more stable and less likely
to dissociate into its constituent ions.
Ionic compounds with large
lattice enthalpies tend to have high melting and boiling points, as significant
energy is needed to break the strong ionic bonds in the solid lattice. These
compounds are generally stable at room temperature and conditions found on
Earth's surface.
In summary, lattice enthalpy
is a measure of the stability of an ionic compound, with higher values
indicating greater stability due to stronger electrostatic forces between the
ions in the lattice.
Q.19.What are the conditions favourable
for intramolecular hydrogen bonding?
Ans. Intramolecular hydrogen bonding occurs when a hydrogen
atom is bonded to an electronegative atom (usually nitrogen, oxygen, or
fluorine) within the same molecule, and there is a second electronegative atom
with a lone pair of electrons in close proximity to the hydrogen. For
intramolecular hydrogen bonding to be favorable, the following conditions are
typically required:
Presence
of Electronegative Atoms: The
molecule should contain electronegative atoms like nitrogen, oxygen, or
fluorine capable of forming hydrogen bonds with a hydrogen atom.
Lone
Pair of Electrons: The
electronegative atom involved in hydrogen bonding should have a lone pair of
electrons available for donation to the hydrogen atom.
Proximity
of Functional Groups: The
hydrogen atom and the electronegative atom with the lone pair of electrons must
be located in the same molecule and in close proximity to each other.
Suitable
Geometry: The molecule should
have the appropriate molecular geometry to facilitate the formation of
intramolecular hydrogen bonds.
Polar
Covalent Bonds: The
bond between the hydrogen atom and the electronegative atom should be polar
covalent to create the necessary partial charges for hydrogen bonding.
Intramolecular hydrogen
bonding is typically stronger than intermolecular hydrogen bonding (between
different molecules) because of the shorter distance and stronger interaction
between the atoms involved. It can have significant effects on the physical and
chemical properties of molecules, influencing their structure, stability, and
reactivity. Intramolecular hydrogen bonding is commonly observed in molecules
containing functional groups such as -OH, -NH, and -COOH, among others.
Q.20.What are the consequences of
intramolecular hydrogen bonding Explain?
Ans. The consequences of intramolecular hydrogen bonding can
have significant effects on the properties and behavior of molecules. Some of
the key consequences include:
Enhanced
Stability: Intramolecular
hydrogen bonding can increase the stability of a molecule by forming a stable
three-dimensional structure. The hydrogen bond acts as an internal force that
holds the molecule together, making it less susceptible to breaking or
undergoing chemical reactions.
Altered
Physical Properties: Intramolecular
hydrogen bonding can lead to changes in the physical properties of a molecule.
For example, it can affect the boiling point, melting point, and solubility of
the compound.
Influences
Reactivity: Intramolecular
hydrogen bonding can influence the reactivity of a molecule. The presence of
intramolecular hydrogen bonds can hinder or facilitate certain chemical
reactions, depending on the specific circumstances.
Modified
Molecular Geometry: The
formation of intramolecular hydrogen bonds can lead to changes in the molecular
geometry. It can affect bond angles and bond lengths, resulting in a non-linear
or bent structure.
Biological
Significance: Intramolecular hydrogen
bonding is common in many biological molecules, such as proteins, nucleic acids
(DNA and RNA), and enzymes. It plays a crucial role in the three-dimensional
folding and stability of these biomolecules, influencing their function and
activity.
Conformational
Changes: Intramolecular
hydrogen bonding can cause conformational changes in a molecule. It can affect
the spatial arrangement of functional groups, leading to different conformations
with distinct properties.
Spectroscopic
Effects: Intramolecular
hydrogen bonding can produce characteristic signals in various spectroscopic
techniques such as infrared (IR) and nuclear magnetic resonance (NMR)
spectroscopy. These signals provide valuable information about the presence and
nature of hydrogen bonding in a molecule.
In summary, intramolecular
hydrogen bonding can have diverse consequences, ranging from altered physical
properties and reactivity to enhanced stability and structural changes. Its
presence is essential in understanding the behavior and function of various
molecules, particularly in biological systems where it plays a vital role in
shaping biomolecular structures and interactions.
Q.21.What is electronic theory of
valency?
Ans. Apologies for the repetition. Here's the answer again:
The electronic theory of
valency, also known as the electronic theory of bonding, is a concept in
chemistry that explains the formation of chemical bonds between atoms based on
the sharing or transfer of electrons to achieve a stable electron
configuration.
According to this
theory:
Valence
Electrons: The chemical
properties of an element are determined by its valence electrons, which are the
electrons present in the outermost energy level (valence shell) of an atom.
Octet
Rule: Atoms tend to gain,
lose, or share electrons to achieve a stable electron configuration with eight
valence electrons (except for hydrogen and helium, which achieve stability with
two valence electrons). This is known as the octet rule.
Ionic
Bonding: When an atom loses or
gains electrons, it forms ions with a stable electron configuration. The
electrostatic attraction between oppositely charged ions leads to the formation
of ionic bonds.
Covalent
Bonding: Atoms can also
achieve a stable electron configuration by sharing electrons with other atoms.
This sharing of electrons results in the formation of covalent bonds.
Dative
(Coordinate) Bond: Some
covalent bonds involve one atom donating a pair of electrons to another atom to
form a bond. This is known as a dative or coordinate bond.
The electronic theory of
valency helps explain the chemical reactivity and bonding behavior of elements
and compounds. It provides insights into the formation of stable molecules and
the role of valence electrons in determining the types of chemical bonds that
form between atoms. The theory is fundamental in understanding the principles
of chemical bonding and is widely used to predict and explain the properties
and behavior of various substances.
Q.22. How is ionic bond formed in NaCI?
Ans. An ionic bond is formed in NaCl (sodium chloride) through
the transfer of electrons from the sodium (Na) atom to the chlorine (Cl) atom.
Sodium (Na) is a metal from
Group 1 of the periodic table and has one valence electron in its outermost
energy level (valence shell).
Chlorine (Cl) is a non-metal
from Group 17 (halogens) and has seven valence electrons in its outermost
energy level.
To achieve a stable electron
configuration, sodium needs to lose its one valence electron, while chlorine
needs to gain one electron to fill its valence shell with eight electrons.
Sodium donates its valence
electron to chlorine, creating a sodium ion (Na⁺) with a positive charge and a
chlorine ion (Cl⁻) with a negative charge.
The electrostatic attraction
between the oppositely charged Na⁺ and Cl⁻ ions results in the formation of an
ionic bond, leading to the creation of the compound sodium chloride (NaCl).
The resulting ionic
compound, NaCl, forms a crystal lattice structure in the solid state due to the
strong electrostatic forces between the positively charged sodium ions and the
negatively charged chloride ions. This ionic bond is relatively strong and
requires a significant amount of energy to break, which is why ionic compounds
like NaCl have high melting and boiling points.
Q.23.How to calculate the dipole
moment?
Ans. The dipole moment of a molecule can be calculated using
the following formula:
Dipole Moment (μ) = Charge
(Q) × Distance (r)
Where:
Charge (Q) is the magnitude
of the partial charge on either end of the bond (usually measured in coulombs,
C, or debyes, D).
Distance (r) is the distance
between the centers of the two charges (usually measured in meters, m, or
angstroms, Å).
In practice, the dipole
moment is often expressed in debyes (D), where 1 debye is equal to 3.336 ×
10^-30 C·m.
To calculate the dipole
moment of a molecule, you need to know the partial charges on the atoms
involved in the bond and the distance between these charges. The partial
charges can be determined experimentally or by using theoretical calculations
such as quantum mechanics.
Keep in mind that the dipole
moment is a vector quantity, meaning it has both magnitude and direction. The
direction of the dipole moment points from the more electronegative atom (with
the partial negative charge) towards the less electronegative atom (with the
partial positive charge). For molecules with multiple bonds or dipoles, you may
need to consider the vector sum of individual bond dipoles to determine the
overall dipole moment of the molecule.
Q.24.How is ionic bond formed in NaCI?
Ans. The ionic bond in NaCl (sodium chloride) is formed
through the transfer of electrons from the sodium (Na) atom to the chlorine
(Cl) atom.
Here's a step-by-step
explanation of how the ionic bond is formed in NaCl:
Sodium (Na) is a metal from
Group 1 of the periodic table and has one valence electron in its outermost
energy level (valence shell).
Chlorine (Cl) is a non-metal
from Group 17 (halogens) and has seven valence electrons in its outermost
energy level.
Sodium wants to achieve a
stable electron configuration similar to the noble gas configuration of neon
(Ne), which has eight valence electrons. To achieve this stability, sodium
needs to lose its one valence electron.
Chlorine wants to achieve a
stable electron configuration similar to the noble gas configuration of argon
(Ar), which also has eight valence electrons. To achieve this stability, chlorine
needs to gain one electron.
In the process of bonding,
sodium loses its one valence electron, becoming a positively charged ion known
as a cation (Na⁺), as it now has one less electron than protons.
The lost electron is gained
by chlorine, which becomes a negatively charged ion known as an anion (Cl⁻), as
it now has one more electron than protons.
The electrostatic attraction
between the oppositely charged Na⁺ and Cl⁻ ions results in the formation of an
ionic bond.
The resulting compound is
sodium chloride (NaCl), with a crystal lattice structure in the solid state due
to the strong electrostatic forces between the positively charged sodium ions
and the negatively charged chloride ions.
In summary, the ionic bond
in NaCl is formed through the complete transfer of one electron from sodium to
chlorine, resulting in the formation of oppositely charged ions that are held
together by strong electrostatic forces, leading to the stable compound sodium
chloride.
Q.25. How to calculate the dipole
moment?
Ans. To calculate the dipole moment of a molecule, you need to
consider the individual bond dipole moments and their orientations in the
molecule. The dipole moment is a vector quantity, meaning it has both magnitude
and direction. Here's how you can calculate the dipole moment:
Determine
Bond Dipole Moments: For
each bond in the molecule, calculate the bond dipole moment. The bond dipole
moment (μ) is the product of the charge (Q) and the distance (r) between the two
atoms involved in the bond.
μ = Q × r
The charge (Q) is the
partial charge on each end of the bond, and the distance (r) is the distance
between the centers of the positive and negative charges.
Consider
Molecular Geometry: Take
into account the molecular geometry and the orientation of individual bond
dipole moments relative to each other. If the bond dipole moments are of equal
magnitude and point in opposite directions, they may cancel each other out,
resulting in a non-polar molecule with a net dipole moment of zero. If the bond
dipole moments do not cancel out, the molecule will have a net dipole moment,
making it polar.
Vector Sum: If the molecule
has multiple polar bonds, determine the vector sum of all the bond dipole
moments to find the net dipole moment of the molecule. This involves
considering the magnitude and direction of each bond dipole moment and adding
them up as vectors.
Expressing
Dipole Moment: The dipole moment is
typically expressed in debyes (D), where 1 debye is equal to 3.336 × 10^-30
C·m. If the dipole moment is zero, the molecule is non-polar, and if it has a
non-zero value, the molecule is polar.
Keep in mind that
calculating the dipole moment can be more complex for larger molecules or
molecules with complicated geometries. Advanced computational methods, such as
quantum mechanics, are often used to calculate the dipole moment accurately in
such cases. For simple molecules, the dipole moment can often be estimated
based on the electronegativities of the atoms involved and the molecular
geometry.