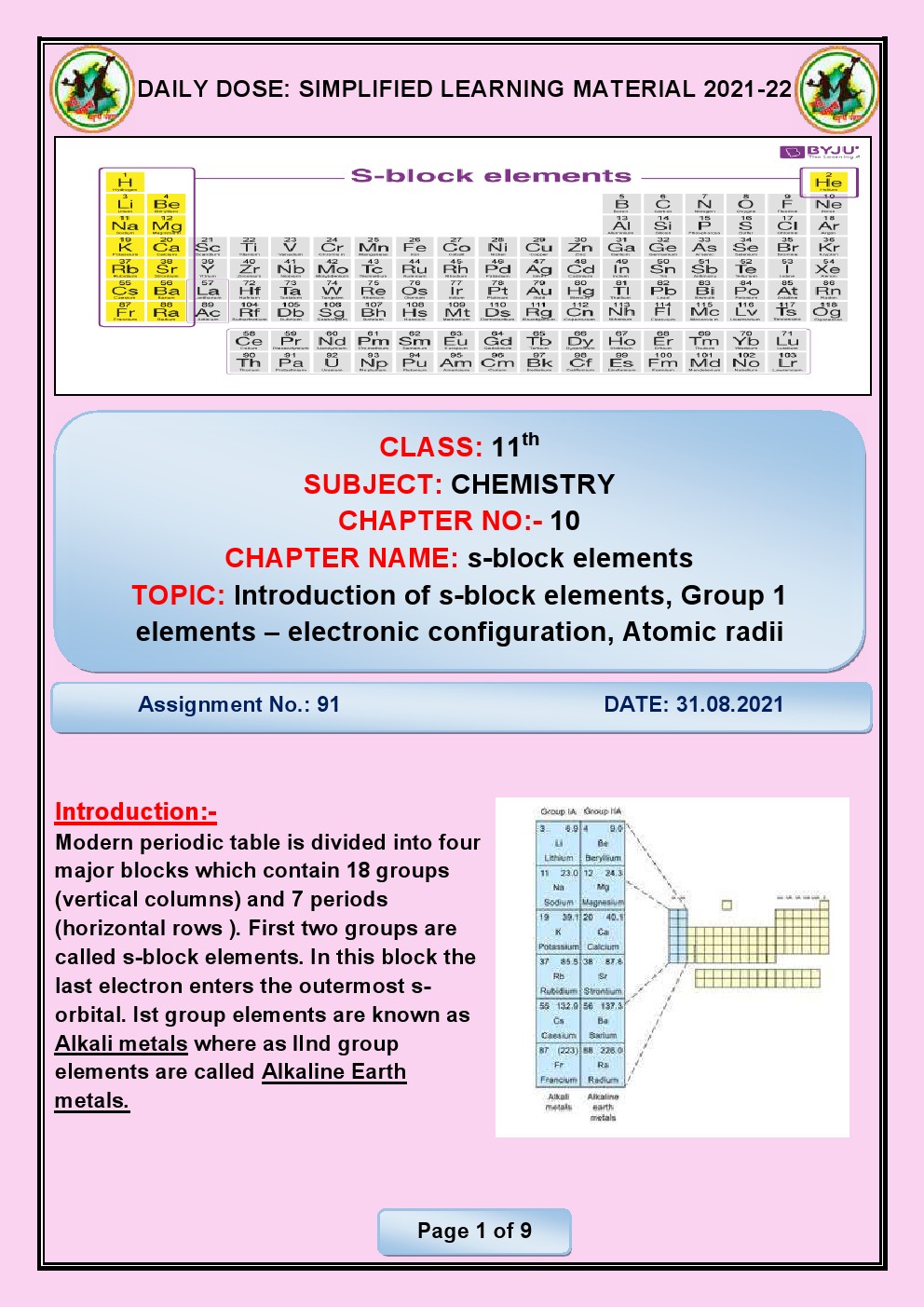
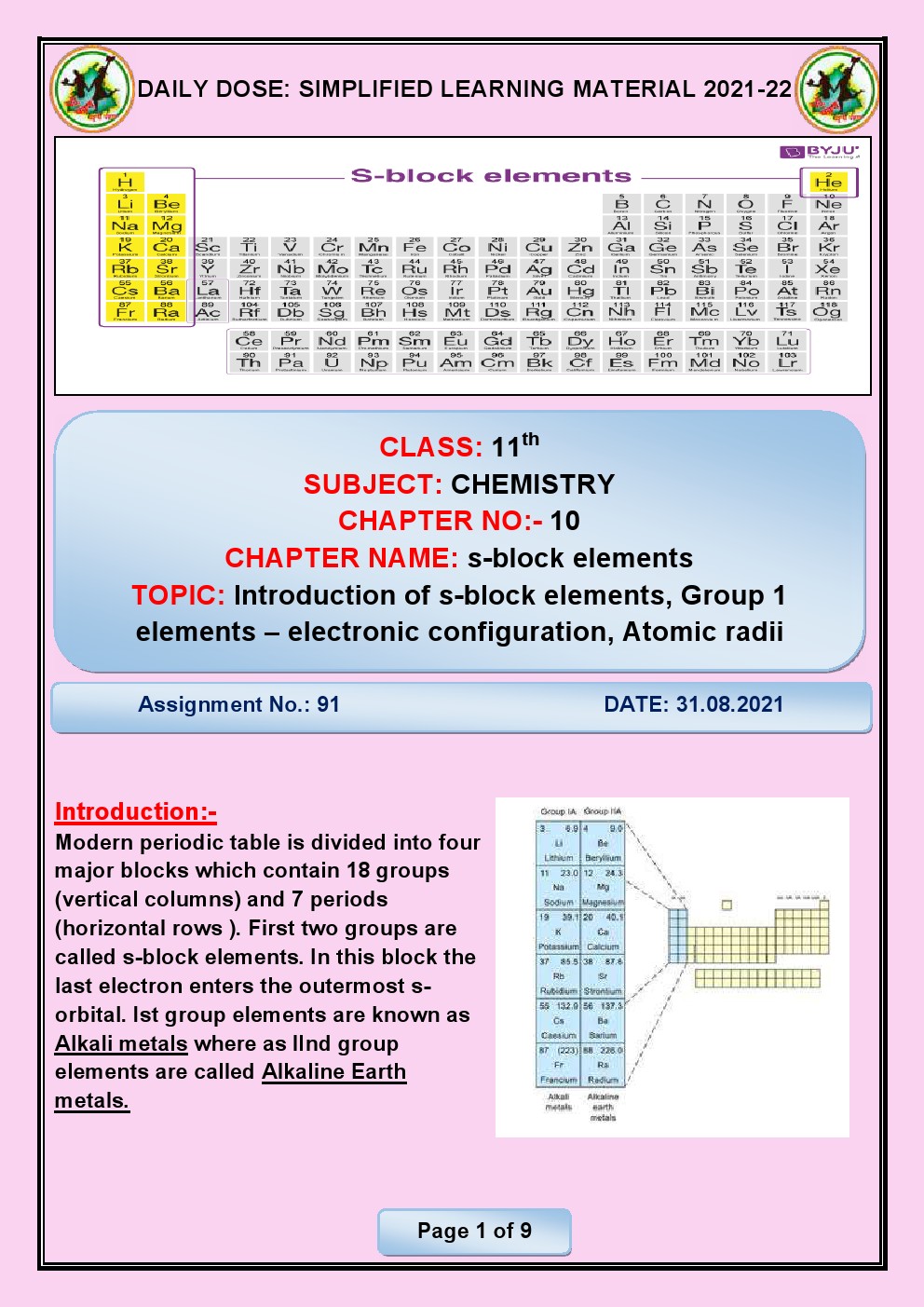
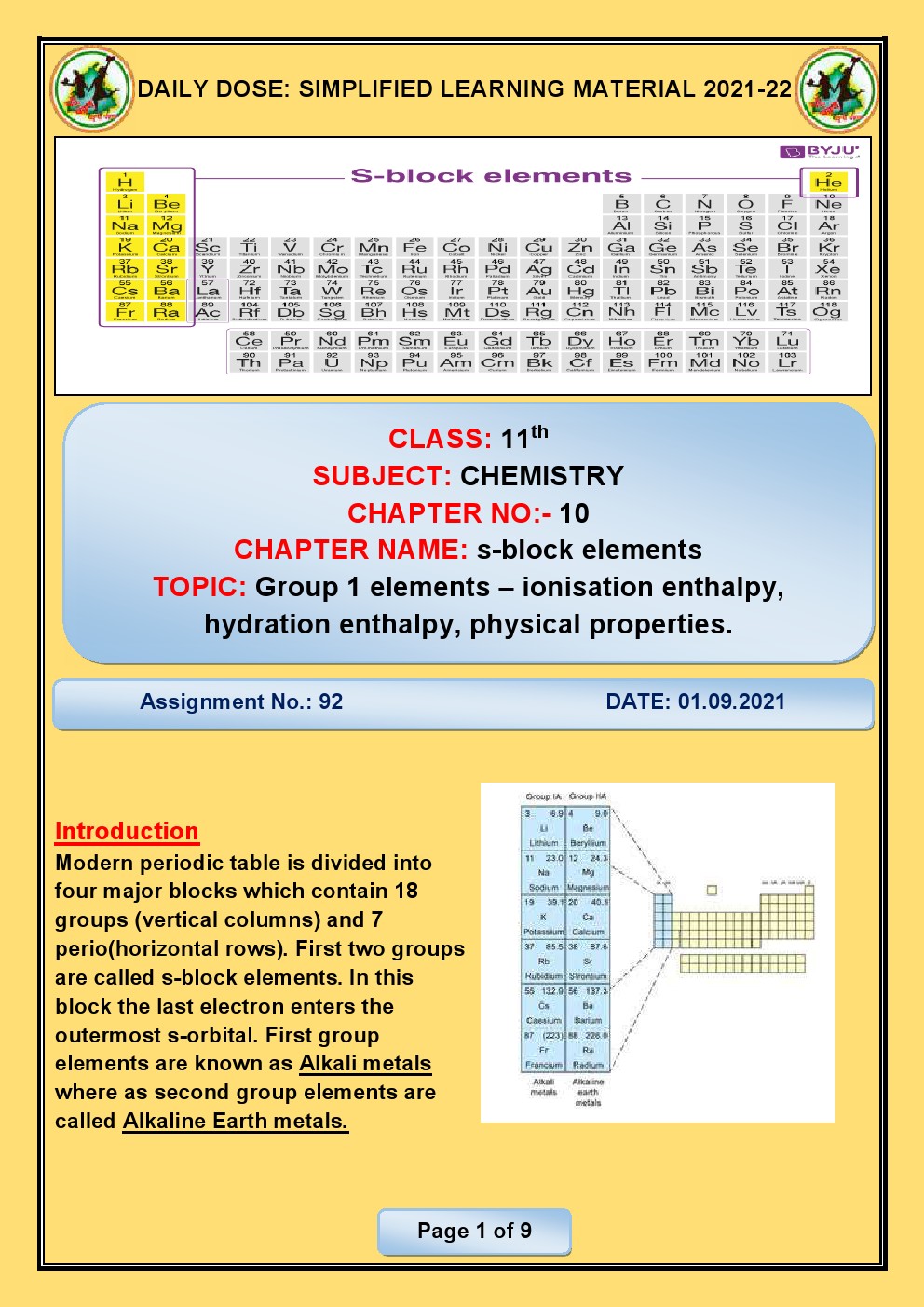
CHAPTER 6 THE S- BLOCK ELEMENTS
VERY SHORT QUESTIONS
ANSWER
Q.1. Why are the elements of group I
called alkali metals?
Ans. Because they react with water to form alkaline (basic)
solutions.
Q.2. Group I elements form largely
univalent ions Explain?
Ans. Group I elements have one valence electron, which they
readily lose to form univalent (monovalent) ions with a charge of +1.
Q.3. Group I elements are strong
reducing agents why?
Ans. Group I elements are strong reducing agents because they
readily lose their valence electron, which can easily donate or transfer
electrons to other elements in chemical reactions.
Q.4.Name the alkali metal which shows
diagonal relationship with magnesium?
Ans. Lithium.
Q.5. Why is LIF insoluble in water?
Ans. Lithium fluoride (LiF) is insoluble in water due to its
high lattice energy and strong ionic bonds between lithium and fluoride ions.
Q.6. Name the alkaline earth metal
hydroxide which is amphoteric?
Ans. Beryllium hydroxide (Be(OH)2) is amphoteric.
Q.7.What is dead burnt plaster?
Ans. Dead burnt plaster, or plaster of Paris, is a dry powder
obtained by heating gypsum to remove its water content, used in various
applications like construction, art, and medicine.
Q.8.Which compound is formed by solvay
process?
Ans. Sodium bicarbonate (NaHCO3) is formed by the Solvay
process.
Q.9. Write the names and symbols of all
the elements of groupI?
Ans. Group I elements are alkali metals. Their names and
symbols are:
Lithium (Li)
Sodium (Na)
Potassium (K)
Rubidium (Rb)
Cesium (Cs)
Francium (Fr)
Q.10.What is the nature of compounds of
lithium?
Ans. The compounds of lithium are mostly ionic in nature.
Q.11. Name the alkali metal which is
radioactive?
Ans. Francium (Fr) is the alkali metal that is radioactive.
Q.12. Group I metals are highly reactive
why?
Ans. Group I metals are highly reactive due to their low ionization
energy and the presence of one valence electron, which they readily lose to
achieve a stable electron configuration.
Q.13. Alkali metal ions are diamagnetic
and colourless Explain?
Ans. Alkali metal ions are diamagnetic (no unpaired electrons)
and colorless because they do not absorb visible light.
Q.14. Alkali metal ions are diamagnetic
and colourless Explain?
Ans. Alkali metal ions are diamagnetic because they have all
their electrons paired up in their electronic configurations, and they are colorless
because they do not absorb visible light.
Q.15. Sodium metal can be used for
drying ether but cannot be used for drying ethanol why?
Ans. Sodium metal can be used for drying ether because it does
not react with it, but it cannot be used for drying ethanol because it reacts
with ethanol, producing undesirable side reactions.
Q.16.Which metal is present in
chlorophyll?
Ans. Magnesium is the metal present in chlorophyll.
Q.17. How does the basic strength of
hydroxides of group II elements vary in a group?
Ans. The basic strength of hydroxides of Group II elements
increases down the group.
Q.18. Why is sodium metal kept under
kerosene oil?
Ans. Sodium metal is kept under kerosene oil to prevent its
reaction with air and moisture.
Q.19.A sodium fire in the laboratory is
not extinguished by water why?
Ans. Reactivity.
Q.20. Give the chemical formulae of
dolomite and carnallite?
Ans. The chemical formulae of dolomite and carnallite are:
Dolomite: CaMg(CO3)2
Carnallite: KMgCl3·6H2O
Q.21. Why lithium exhibits anomalous
behaviour?
Ans. Lithium exhibits anomalous behavior due to its small
atomic size and high charge-to-size ratio, leading to unique properties
compared to other elements in the alkali metal group.
Q.22. Why is gypsum added to powdered
clinkers to get cement?
Ans. Gypsum is added to powdered clinkers to regulate the
setting time of cement and control its early hydration process.
Q.23. Name the insoluble fluorides of
group I and group II?
Ans. Insoluble fluorides of Group I and Group II:
Group I - None
Group II - Calcium fluoride
(CaF2)
Q.24.Give the chemical formula of
storel’s cement?
Ans. The chemical formula of Storel's cement is
4CaO·Al2O3·Fe2O3·SO3.
Q.25.What is soda ash?
Ans. Soda ash is sodium carbonate (Na2CO3), a white powder
used in industries like glassmaking and cleaning products.
Q.26.What is magnesia cement or storel’s
cement?
Ans. Magnesia cement or Storel's cement is a type of cement
formed by the reaction of magnesium oxide (MgO) with a suitable proportion of
water to produce a dense, high-strength material.
Q.27.What is fluid magnesia?
Ans. Fluid magnesia is a highly concentrated magnesium
hydroxide suspension used as an antacid and laxative.
Q.28.What is hydrolith?
Ans. Hydrolith is an impure form of calcium chloride obtained
from the reaction of calcium hydroxide with ammonium chloride.
Q.29.Why do deryllium halides fume in
moist air?
Ans. Beryllium halides fume in moist air due to their
hygroscopic nature, absorbing moisture from the air and forming a solution that
evaporates as visible fumes.
Q.30. Arrange the following in
increasing order of solubility on water?
Ans. The order of increasing solubility in water is: Mg(OH)2
< Be(OH)2 < Ca(OH)2.
SHORT QUESTIONS ANSWER
Q.1. Group I elements are poor
complexing agents Explain?
Ans. Group I elements are poor complexing agents because they
have large atomic radii and low charge densities, which result in weak
interactions with ligands, making it difficult for them to form stable
complexes with other molecules.
Q.2.The alkali metals have the lowest
first ionization energies in their respective periods why?
Ans. The alkali metals have the lowest first ionization
energies in their respective periods due to their large atomic size and low
effective nuclear charge, making it easier to remove the outermost electron.
Q.3.The alkali metals are soft low melting
and have low densities give reasons?
Ans. The alkali metals are soft, low melting, and have low
densities because their atoms have a single valence electron, which experiences
weak metallic bonding. This results in a relatively low force of attraction
between atoms, making them easily deformable, low melting points, and low
densities
Q.4. Alkali metals show characteristics
colours when introduced into a Bunsen‘s flame Explain?
Ans. When alkali metals are introduced into a Bunsen flame,
they show characteristic colors due to the excitation and subsequent relaxation
of their valence electrons. When heated in the flame, the outermost electron of
the alkali metal atoms gets excited to higher energy levels. As the excited
electron returns to its ground state, it releases the excess energy in the form
of light. Each alkali metal emits a unique set of wavelengths of light,
producing specific colors in the flame. For example:
Lithium: Crimson red
Sodium: Yellow
Potassium: Lilac
Rubidium: Red-violet
Cesium: Blue-violet
These colors are used as a
qualitative test to identify the presence of specific alkali metals in
compounds or mixtures.
Q.5.The second inonisation energy of
alkali metals is very high as compared to its ionization energy Give reasons.
Ans. The second ionization energy of alkali metals is very
high compared to their first ionization energy due to the removal of the second
valence electron. After losing the first valence electron, the remaining
electron experiences a stronger effective nuclear charge, making it more
difficult to remove the second electron. This is because the loss of the first
electron results in a change in the electronic configuration, leading to a more
stable configuration for the remaining electron. As a result, it requires
significantly more energy to remove the second electron, resulting in a higher
second ionization energy.
Q.6.Chemical reactivity of the alkali
metals increases from Li to Cs. Explain?
Ans. The chemical reactivity of alkali metals increases from
Li to Cs due to the following factors:
Atomic
Size: As we move down the
group from lithium (Li) to cesium (Cs), the atomic size increases. The increase
in atomic size leads to a decrease in the effective nuclear charge felt by the
outermost electron, making it easier to remove. This results in a lower
ionization energy and higher reactivity.
Electronegativity: The electronegativity of alkali metals decreases down the
group. This means that as we move from Li to Cs, the atoms become less likely
to hold onto their valence electrons, making them more reactive.
Electron
Configuration: The alkali metals all
have one valence electron in their outermost shell. As we move down the group,
the additional electron shells provide more shielding and reduce the attraction
between the valence electron and the nucleus, making it easier for the outer
electron to be involved in chemical reactions.
Metallic
Character: The metallic
character of alkali metals increases down the group. This means that they have
a greater tendency to lose electrons and form positive ions, increasing their
reactivity in chemical reactions.
These factors collectively
contribute to the increasing chemical reactivity of alkali metals from Li to
Cs. Cesium (Cs) is the most reactive alkali metal due to its larger atomic
size, low ionization energy, and high metallic character.
Q.7. Unlike other alkali metals
carbonates and hydroxides of lithium are thermally less stable?
Ans. That's correct. Lithium carbonates and hydroxides are
thermally less stable compared to other alkali metal carbonates and hydroxides.
The thermal stability of
alkali metal carbonates and hydroxides generally increases down the group from
lithium (Li) to cesium (Cs). Lithium carbonate (Li2CO3) and lithium hydroxide
(LiOH) are exceptions to this trend and are less stable compared to the
corresponding compounds of other alkali metals.
The lower thermal stability
of lithium carbonates and hydroxides is primarily due to the smaller size of
the lithium cation and the higher charge-to-size ratio. These factors result in
stronger electrostatic attractions between the lithium cation and the carbonate
or hydroxide anion, making it easier for the compounds to decompose upon
heating.
For example, lithium carbonate
decomposes at a lower temperature compared to other alkali metal carbonates,
forming lithium oxide (Li2O) and carbon dioxide (CO2):
2Li2CO3(s) → Li2O(s) +
2CO2(g)
Similarly, lithium hydroxide
decomposes at a lower temperature compared to other alkali metal hydroxides, forming
lithium oxide and water:
2LiOH(s) → Li2O(s) + H2O(g)
This lower thermal stability
of lithium carbonates and hydroxides has implications in various industrial and
chemical processes.
Q.8. Why is LICI soluble in organic
solvents?
Ans. Lithium chloride (LiCl) is soluble in organic solvents
because it forms strong ion-dipole interactions with polar organic molecules. The
small size and high charge density of the lithium cation allow it to have
strong electrostatic attractions with the partially negative charges of polar
solvent molecules. This enables lithium chloride to dissolve and form stable
solutions in various organic solvents.
Q.9. Why do alkali metals not form M2+
ions?
Ans. Alkali metals do not form M2+ ions because they have a
single valence electron in their outermost electron shell. To achieve a stable
electron configuration, alkali metals prefer to lose this one valence electron,
forming M+ ions with a charge of +1. Forming M2+ ions would require the loss of
two valence electrons, which would result in a less stable electronic
configuration and require significantly higher energy, making it unfavorable
for alkali metals to exist as M2+ ions. As a result, alkali metals
predominantly form monovalent (M+) ions in chemical reactions.
Q.10. Why are the alkali metals the
most electropositive in nature?
Ans. Alkali metals are the most electropositive in nature
because they have the lowest ionization energies among all elements. The
ionization energy is the energy required to remove an electron from an atom in
its gaseous state. Alkali metals have a single valence electron in their
outermost electron shell, and due to their large atomic size and low effective
nuclear charge, this electron is loosely held and easily removed. As a result,
alkali metals readily lose their valence electron to form positive ions
(cations) with a charge of +1. This strong tendency to lose electrons and form
cations makes alkali metals highly electropositive, exhibiting their metallic
nature and reactivity in various chemical reactions.
Q.11. Why is the melting point of
sodium lesser than than that of lithium?
Ans. The melting point of sodium is lower than that of lithium
because of the difference in the strength of metallic bonding between the two
elements.
In metallic bonding, the
positively charged metal ions are held together by a "sea" of
delocalized electrons. The strength of this metallic bonding depends on factors
like the charge of the metal ions and the number of delocalized electrons.
Lithium has a smaller atomic
size and a higher charge-to-size ratio compared to sodium. Due to this, the
lithium ions are more strongly attracted to the delocalized electrons,
resulting in stronger metallic bonding. This stronger bonding requires higher
energy to break the forces holding the metal ions together, leading to a higher
melting point.
On the other hand, sodium
has a larger atomic size and a lower charge-to-size ratio, which results in
weaker metallic bonding. This weaker bonding requires less energy to overcome,
leading to a lower melting point compared to lithium.
Therefore, the difference in
the strength of metallic bonding is the reason for the lower melting point of
sodium compared to lithium.
Q.12.What will happen when crystalline
washing soda is exposed to air?
Ans. When crystalline washing soda (sodium carbonate
decahydrate - Na2CO3·10H2O) is exposed to air, it will undergo a process called
efflorescence. Efflorescence occurs when the water of crystallization in the
washing soda crystals evaporates, leaving behind a white, powdery residue on
the surface of the crystals.
The process of efflorescence
happens because the air surrounding the washing soda crystals is not completely
dry, and it contains some moisture. As the air comes into contact with the
crystals, the moisture in the air dissolves the water of crystallization from
the washing soda. As the moisture evaporates, it leaves behind the anhydrous
form of sodium carbonate (Na2CO3), which is a white, powdery substance.
The reaction is as
follows:
Na2CO3·10H2O (crystalline washing
soda) + H2O (moisture in air) → Na2CO3 (anhydrous sodium carbonate) + 10H2O
(evaporated water)
This efflorescence process
is common in substances that contain water of crystallization, and it results
in the formation of a white, powdery deposit on the surface of the crystals.
Q.13. Why are salts of alkali metals
diamagnetic in nature?
Ans. Salts of alkali metals are diamagnetic in nature because
they have all their electrons paired up in their electronic configurations.
Diamagnetic substances have no unpaired electrons, which means they do not
possess any net magnetic moment.
In alkali metals, like
lithium (Li), sodium (Na), potassium (K), etc., the valence electron is easily
lost during the formation of salts, resulting in the formation of cations with
a completely filled electron configuration. These cations have all their
electrons paired up, leading to diamagnetic behavior.
Since there are no unpaired
electrons in the electronic configurations of alkali metal salts, they do not
exhibit any magnetic properties and are diamagnetic.
Q.14. Why is LIH more stable than NAH?
Ans. Lithium hydride (LiH) is more stable than sodium hydride
(NaH) due to the smaller size and higher charge-to-size ratio of lithium
compared to sodium.
In LiH, the lithium cation (Li+)
and hydride anion (H-) are held together by strong ionic bonds. The small size
of the lithium cation allows for a closer approach of the positive and negative
charges, resulting in stronger electrostatic attractions between the ions,
making LiH more stable.
In NaH, the sodium cation
(Na+) and hydride anion (H-) are also held together by ionic bonds. However,
the larger size of the sodium cation compared to lithium leads to weaker
electrostatic attractions between the ions, making NaH less stable than LiH.
Additionally, lithium and
hydrogen have a higher electronegativity difference compared to sodium and
hydrogen. This higher electronegativity difference in LiH results in a more
covalent character in its bonding, further enhancing its stability.
Overall, the smaller size,
higher charge-to-size ratio, and greater electronegativity difference make LiH
more stable than NaH.
Q.15. Why does beryllium show
resemblance with aluminium?
Ans. Beryllium shows resemblance with aluminum due to their
similar electronic configurations and their tendency to form covalent bonds.
Both beryllium and aluminum
are in Group 2 and Group 13 of the periodic table, respectively. They both have
two and three valence electrons, respectively, in their outermost electron
shells. The similarity in their electronic configurations (Be: 2s^2 and Al:
3s^2 3p^1) gives them certain chemical similarities.
Additionally, both beryllium
and aluminum have a relatively high charge-to-size ratio, which results in a
greater tendency to form covalent bonds rather than ionic bonds. Beryllium has
a small atomic size and a high charge (+2), while aluminum has a small atomic
size and a moderate charge (+3). This leads to the formation of covalent
compounds in both elements.
Furthermore, beryllium oxide
(BeO) and aluminum oxide (Al2O3) are amphoteric oxides, meaning they can act as
both acids and bases, and both elements form covalent hydrides (BeH2 and AlH3).
These similarities in
electronic configuration, charge-to-size ratio, and bonding behavior make beryllium
exhibit some resemblance with aluminum in certain chemical properties and
reactions.Q.16.Why is second ionsation energy of Na more than Mg?
Q.16. Why second ionsation energy of Na
more than Mg?
Ans. The second ionization energy of sodium (Na) is greater
than that of magnesium (Mg) because of the electron configuration and the stability
of the resulting ions.
In the first ionization of
sodium, one electron is removed from the 3s^1 electron configuration, resulting
in a stable sodium ion (Na+):
Na → Na+ + e^-
After the first ionization,
the electronic configuration of sodium becomes 2s^2 2p^6, which is the same as
the electron configuration of the noble gas neon (Ne). This stable electronic
configuration makes it energetically unfavorable to remove another electron
from the sodium ion, resulting in a higher second ionization energy.
On the other hand, in the
first ionization of magnesium, one electron is removed from the 3s^2 electron
configuration:
Mg → Mg+ + e^-
The resulting magnesium ion
(Mg+) has the electronic configuration of 2s^2 2p^6, which is not as stable as
the noble gas electron configuration. As a result, the removal of a second
electron from the magnesium ion is energetically more favorable compared to
sodium, leading to a lower second ionization energy for magnesium.
In summary, the difference
in the electron configuration and the stability of the resulting ions leads to
the second ionization energy of sodium being greater than that of magnesium.
Q.17. Why do alkaline earth metals act
as reducing agent?
Ans. Alkaline earth metals act as reducing agents because they
have a strong tendency to lose their valence electrons. In chemical reactions,
reducing agents are substances that donate electrons to other species, causing
the reduction (gain of electrons) of the other species.
Alkaline earth metals, such
as calcium (Ca), strontium (Sr), and barium (Ba), have two valence electrons in
their outermost electron shell. These electrons are loosely held due to the
relatively large atomic size and low effective nuclear charge. As a result,
alkaline earth metals readily lose their valence electrons to form stable
cations with a charge of +2.
When they react with other
substances, such as metal oxides or metal ions in a compound, alkaline earth
metals donate their valence electrons to reduce the other species. For example,
they can reduce metal oxides to form pure metals:
Ca + Fe2O3 → CaO + Fe
In this reaction, calcium
(Ca) acts as a reducing agent by donating electrons to iron oxide (Fe2O3) to
produce calcium oxide (CaO) and pure iron (Fe).
Due to their strong reducing
properties, alkaline earth metals are used in various industrial processes and
metallurgical applications for the extraction of metals and reduction
reactions.
Q.18.What is the biological importance
of calcium?
Ans. Calcium is biologically important for various vital
functions in living organisms:
Bone
and Teeth Formation: Calcium
is a crucial component of bones and teeth, providing strength and rigidity to
the skeletal structure. It is essential for bone development and maintenance,
ensuring proper growth and strength throughout life.
Muscle
Contraction: Calcium plays a vital
role in muscle contraction. When a muscle receives a signal to contract,
calcium ions are released from the sarcoplasmic reticulum inside muscle cells,
triggering the muscle fibers to contract.
Nerve
Function: Calcium is involved
in nerve transmission and signaling. It helps in the release of
neurotransmitters, which are essential for transmitting signals between nerve
cells and enabling proper communication within the nervous system.
Blood
Clotting: Calcium is essential
for blood clotting (coagulation). When there is a wound or injury, calcium ions
interact with various clotting factors to form a blood clot, preventing
excessive bleeding.
Enzyme
Activation: Calcium acts as a
cofactor for several enzymes, playing a crucial role in activating and
regulating their activity. Enzymes are essential for various metabolic
processes in the body.
Cell
Signaling: Calcium ions are
involved in various cellular signaling pathways, influencing cell growth,
proliferation, and differentiation. They help regulate cell functions and
responses to external stimuli.
Hormone
Secretion: Calcium is necessary
for the secretion of several hormones, including insulin from the pancreas and
various hormones from endocrine glands.
Cellular
Adhesion: Calcium plays a role
in cell-to-cell adhesion and maintaining the integrity of cell membranes,
contributing to tissue structure and stability.
Cardiovascular
Health: Calcium is important
for maintaining the normal function of the heart and blood vessels, including
maintaining the correct rhythm of the heart and promoting healthy blood pressure.
Overall, calcium is an
essential mineral required for the proper functioning and health of various
systems in the body, and its balance is crucial for overall well-being and
physiological functions.
Q.19. Write the nature of beryllium
oxide?
Ans. Beryllium oxide (BeO) is an ionic compound with a high
melting point and a high electrical and thermal conductivity. It has a
ceramic-like nature and is chemically inert, making it useful in high-temperature
applications such as in nuclear reactors and semiconductor devices.
Q.20.Mention the trend in thermal stability
of carbonates of alkaline earth metals?
Ans. The trend in thermal stability of carbonates of alkaline
earth metals is that it increases down the group.
As we move down the alkaline
earth metal group from beryllium (Be) to barium (Ba), the thermal stability of
their carbonates increases. This is due to the larger size and higher
polarizability of the metal ions as we move down the group. The larger metal
ions can better stabilize the carbonate ion, leading to stronger ionic bonds
between the metal cation and carbonate anion. As a result, the carbonates of
alkaline earth metals become more thermally stable with increasing atomic
number down the group.
Q.21. Write the trend in the solubility
of fluorides of alkaline earth metals in water?
Ans. The trend in the solubility of fluorides of alkaline
earth metals in water is that it decreases down the group.
As we move down the alkaline
earth metal group from beryllium (Be) to barium (Ba), the solubility of their
fluorides in water decreases. This trend is mainly due to the decreasing
charge-to-size ratio of the metal cations as we move down the group.
Beryllium fluoride (BeF2)
and magnesium fluoride (MgF2) are both sparingly soluble in water. As we move
down the group, the ionic radius of the metal cations increases while the
charge remains the same. This leads to a decrease in the charge-to-size ratio
of the metal cations, making them less capable of attracting and interacting
with water molecules.
As a result, calcium
fluoride (CaF2) and strontium fluoride (SrF2) have even lower solubility in
water than beryllium fluoride and magnesium fluoride. Barium fluoride (BaF2) is
the least soluble among the fluorides of alkaline earth metals.
In summary, the solubility
of fluorides of alkaline earth metals in water decreases from beryllium
fluoride to barium fluoride as we move down the group.
Q.22. Why is magnesium oxide used for
lining of steel-making furnaces?
Ans. Magnesium oxide (MgO) is used for lining steel-making
furnaces due to its excellent refractory properties and resistance to high
temperatures.
The main reasons for using
magnesium oxide as a refractory lining material in steel-making furnaces are:
High
Melting Point: Magnesium oxide has a
very high melting point (around 2800°C), making it capable of withstanding the
extremely high temperatures reached inside steel-making furnaces without
melting or deforming.
Heat
Resistance: It has excellent heat
resistance, allowing it to maintain its structural integrity even under intense
heat and thermal cycling.
Low
Thermal Expansion: Magnesium
oxide has a low coefficient of thermal expansion, meaning it expands and
contracts very little with temperature changes. This property helps prevent the
lining from cracking or becoming weak due to temperature variations during
furnace operation.
Corrosion
Resistance: Magnesium oxide is
resistant to the corrosive effects of molten steel and other metal oxides
present in the furnace during steel-making processes.
Insulating
Properties: It has good
insulating properties, which helps maintain high temperatures inside the
furnace and conserves energy.
Mechanical
Strength: Magnesium oxide
provides good mechanical strength, which is essential to withstand the
mechanical stress experienced during charging, tapping, and other operations in
the steel-making process.
Due to these beneficial
properties, magnesium oxide is a preferred material for lining steel-making
furnaces, including basic oxygen furnaces and electric arc furnaces, where high
temperatures and corrosive environments are prevalent.
Q.23. Why is the third ionisation
enthalpy of alkaline earth metals much more than the second ionisation energy?
Explain?
Ans. The third ionization enthalpy of alkaline earth metals is
much more than the second ionization energy due to the removal of an electron
from a stable noble gas electron configuration.
When an alkaline earth metal
loses its first two valence electrons, it forms a stable divalent cation with a
noble gas electron configuration. For example, in the case of calcium (Ca):
Ca → Ca^2+ + 2e^-
After losing two electrons,
the electronic configuration of Ca^2+ is the same as the noble gas argon (Ar),
which has a fully filled electron shell. This configuration is highly stable,
and the cation becomes less likely to lose another electron, as it would result
in an unstable electron configuration.
The third ionization
enthalpy is the energy required to remove an electron from this stable divalent
cation to form a trivalent cation:
Ca^2+ → Ca^3+ + e^-
Since the noble gas electron
configuration of the divalent cation is much more stable than a trivalent
cation, it requires significantly more energy to remove the third electron.
This results in a much higher third ionization enthalpy compared to the second
ionization energy.
In general, as we move down
the alkaline earth metal group, the ionization enthalpies increase due to the
increase in effective nuclear charge and the smaller size of the cations.
However, the jump from the second to the third ionization enthalpy is more
significant due to the stability of the noble gas electron configuration
attained after the loss of the first two valence electrons.
Q.24. Why are carbonates of alkali
metals thermally more stable than alkaline earth metals?
Ans. Carbonates of alkali metals are thermally more stable
than alkaline earth metals due to the difference in the size and charge of the
cations.
The stability of a carbonate
depends on the strength of the ionic bond between the metal cation and the
carbonate anion. In carbonates, the carbonate ion (CO3^2-) has a charge of -2,
and it forms an ionic bond with the metal cation.
In alkali metal carbonates
(Li2CO3, Na2CO3, K2CO3, etc.), the metal cations (Li+, Na+, K+) have a
relatively larger ionic size and lower charge density. Due to this, the
electrostatic attraction between the metal cation and the carbonate anion is
weaker, resulting in a less stable ionic bond. As a result, alkali metal
carbonates are relatively less thermally stable and decompose at lower
temperatures to form metal oxides and carbon dioxide:
2Li2CO3(s) → Li2O(s) + 2CO2(g)
In alkaline earth metal
carbonates (MgCO3, CaCO3, SrCO3, BaCO3, etc.), the metal cations (Mg^2+, Ca^2+,
Sr^2+, Ba^2+) have a smaller ionic size and higher charge density compared to
alkali metal cations. This leads to stronger electrostatic attractions between
the metal cation and the carbonate anion, resulting in a more stable ionic
bond. As a result, alkaline earth metal carbonates are more thermally stable
and require higher temperatures to decompose:
CaCO3(s) → CaO(s) + CO2(g)
In summary, the larger size
and lower charge density of alkali metal cations result in weaker ionic bonds
in their carbonates, making them less thermally stable compared to alkaline
earth metal carbonates, where the smaller size and higher charge density of the
cations lead to stronger ionic bonds and higher thermal stability.
Q.25. Why has lithium ion the lowest
and caesium ion the highest mobility in an electrie field?
Ans. The mobility of ions in an electric field depends on
their charge and size. In the case of lithium ion (Li+) and caesium ion (Cs+),
their mobilities are influenced by the following factors:
Charge: Both Li+ and Cs+ ions are monovalent cations, meaning
they have a charge of +1. Since their charges are the same, this factor does
not contribute to the difference in their mobilities.
Size: The size of ions also plays a significant role in their
mobility. Li+ ion is relatively small in size, as it has fewer electron shells
and a higher effective nuclear charge. Cs+ ion, on the other hand, is much
larger due to the presence of more electron shells and a lower effective
nuclear charge.
Due to its smaller size, Li+
ion experiences stronger electrostatic forces with the surrounding ions and
molecules. This results in greater resistance to movement in the electric
field, leading to lower mobility.
Conversely, Cs+ ion, being
larger, experiences weaker electrostatic forces with the surrounding ions and
molecules. This results in less resistance to movement in the electric field,
leading to higher mobility.In summary, the difference in mobility between Li+
and Cs+ ions is primarily due to their size, with Li+ having the lowest
mobility due to its smaller size and Cs+ having the highest mobility due to its
larger size.
Q.26.Why do sodium and potassium not
form complex ions?
Ans. Sodium and potassium do not form complex ions
(coordination complexes) due to their large size and low charge-to-size ratio.
Complex ions are formed when
a central metal ion is surrounded by ligands (molecules or ions with lone pairs
of electrons) that coordinate to the metal ion through dative covalent bonds.
The formation of complex ions requires the metal ion to have a suitable
charge-to-size ratio, which allows it to interact effectively with ligands and
form stable coordination complexes.
Sodium (Na) and potassium
(K) are alkali metals with one valence electron in their outermost electron
shell. When they lose this valence electron, they form monovalent cations (Na+
and K+). These monovalent cations have a large size and a low charge-to-size
ratio, making them less capable of effectively coordinating with ligands to
form stable complex ions.
In contrast, transition
metals and some other metal ions with smaller sizes and higher charge-to-size
ratios are more likely to form complex ions. Transition metals have partially
filled d-orbitals, which allows them to accept electron pairs from ligands and
form coordination complexes with various ligands.
In summary, the large size
and low charge-to-size ratio of sodium and potassium cations make them less
favorable for forming complex ions. They typically do not coordinate
effectively with ligands to form stable coordination complexes as commonly
observed with transition metal ions.
Q.27.Why are salts of alkaline earth
metals diamagnetic in nature?
Ans. Salts of alkaline earth metals are diamagnetic in nature
because they have completely filled electron configurations in their cations.
Diamagnetism is a property
exhibited by substances that have all their electrons paired up in their electronic
configurations. When all the electrons are paired, there is no net magnetic
moment in the substance, and it does not show any attraction to an external
magnetic field.
In the case of alkaline
earth metals, such as beryllium (Be), magnesium (Mg), calcium (Ca), strontium
(Sr), and barium (Ba), they lose their valence electrons to form cations with a
noble gas electron configuration. For example, the electronic configuration of
the divalent cation Mg^2+ is the same as that of neon (Ne):
Mg^2+: 1s^2 2s^2 2p^6
Since all the electrons in
the electronic configuration are paired, these cations are diamagnetic and do
not show any significant magnetic properties. This diamagnetic behavior is a
common characteristic of salts containing alkaline earth metal cations due to
their stable electron configurations.
Q.28. Why is gypsum added to powdered
clinkers to get cement?
Ans. Gypsum (calcium sulfate dihydrate - CaSO4·2H2O) is added
to powdered clinkers to regulate the setting time of cement and control its
early strength development.
During the manufacturing of
cement, clinker is produced by heating a mixture of limestone, clay, and other
raw materials in a kiln at a very high temperature. The resulting clinker is
then ground into a fine powder, which is the main component of cement.
When water is added to
cement, a chemical reaction known as hydration occurs, where the cement
particles react with water to form various calcium-silicate-hydrate (C-S-H)
compounds. This hydration process is responsible for the hardening and setting
of cement.
However, if the hydration
reaction proceeds too rapidly, it can cause the cement to set too quickly,
making it difficult to work with and adversely affecting the final product.
This is especially problematic during transportation and placement of the
cement.
Gypsum is added to the
powdered clinkers to act as a retarding agent. It reacts with the tricalcium
aluminate (C3A) phase in the clinker to form ettringite, which slows down the
hydration process. This retards the setting time of cement and helps to prevent
it from setting too quickly.
By adding gypsum to the
powdered clinkers, cement manufacturers can control the setting time of the
cement and improve its workability, making it easier to handle during
construction while still achieving the desired strength properties in the long
run.
Q.29. Why does the solubility of
alkaline earth metal hydroxides increase down the group?
Ans. The solubility of
alkaline earth metal hydroxides increases down the group due to the increase in
the size and polarizability of the metal cations.
As we move down the alkaline
earth metal group from beryllium (Be) to barium (Ba), the size of the metal
cations increases. This is because the number of electron shells increases as
we go down the group, resulting in larger atomic and ionic radii.
The larger size of the metal
cations in alkaline earth metals down the group leads to two main effects:
Increase
in Polarizability: The
larger metal cations have more electron clouds, making them more easily
polarizable. Polarizability refers to the ability of an ion to distort its
electron cloud in the presence of an electric field. Larger ions can undergo
more significant electron cloud distortions, which allows them to form stronger
ion-dipole interactions with water molecules. This increases the solubility of
the hydroxides in water.
Weakening
of the Lattice Energy: The
lattice energy is the energy required to break the ionic bonds and separate the
cations and anions in a solid ionic compound. As the size of the metal cations
increases, the lattice energy decreases. Larger cations can spread out the
negative charge of the hydroxide ions over a larger volume, reducing the
electrostatic attraction between the cations and hydroxide ions in the solid.
This weakening of the lattice energy makes it easier for the hydroxides to
dissolve in water.
Overall, the increase in
polarizability and the weakening of the lattice energy down the group result in
higher solubility of alkaline earth metal hydroxides in water as we move from
beryllium hydroxide (Be(OH)2) to barium hydroxide (Ba(OH)2).
Q.30.Discuss the composition and
manufacturing in details of cement?
Ans. Cement is a crucial construction material used worldwide
to bind and strengthen various building elements. It is primarily composed of
four main components: calcium, silicon, aluminum, and iron. The two most common
types of cement are Portland cement and blended cement. Below is a detailed
discussion of the composition and manufacturing process of Portland cement:
Composition of
Portland Cement:
Tricalcium
Silicate (C3S): This
compound is the primary contributor to the early strength of cement. It
hydrates rapidly and provides initial strength to the concrete.
Dicalcium
Silicate (C2S): It
hydrates more slowly than C3S but contributes to the long-term strength
development of cement.
Tricalcium
Aluminate (C3A): This
compound contributes to the early setting of cement. However, its high
reactivity can lead to the rapid generation of heat, which can cause cracking in
massive concrete structures.
Tetracalcium
Aluminoferrite (C4AF): It
contributes to the late strength development and imparts a grayish color to the
cement.
Gypsum
(Calcium Sulfate Dihydrate): Gypsum is added to regulate the setting time of cement
and control its early strength development.
Manufacturing Process
of Portland Cement:
The manufacturing process of
Portland cement involves several stages:
Raw
Material Extraction: Limestone,
clay, shale, and other suitable materials are extracted from quarries or mines.
These raw materials are crushed and transported to the cement plant.
Raw
Material Preparation: The
raw materials are further crushed and ground into a fine powder. This powder is
known as raw meal.
Clinker
Production: The raw meal is then
heated in a rotary kiln at very high temperatures (around 1450°C). During this
process, the raw materials undergo a series of chemical reactions to form
small, dark-gray nodules called clinker.
Clinker
Grinding: The clinker is cooled
and ground into a fine powder with the addition of small amounts of gypsum to
control the setting time of cement. This ground clinker, along with gypsum, is
known as cement.
Storage
and Packaging: The finished cement
is stored in silos or large storage tanks. It is then packed into bags or
shipped in bulk for distribution to construction sites.
Quality
Control: Throughout the
manufacturing process, strict quality control measures are applied to ensure
the consistency and quality of the final product.
Blended Cement:
Blended cement is a
variation of Portland cement where a portion of the clinker is replaced with
supplementary cementitious materials (SCMs) like fly ash, slag, or silica fume.
These SCMs enhance the properties of the cement, such as reducing the heat of
hydration, improving durability, and reducing the carbon footprint.
In conclusion, Portland
cement is manufactured through a controlled process involving raw material
extraction, preparation, clinker production, grinding, and final packaging. The
composition of cement and its manufacturing process determine its properties
and suitability for various construction applications.