12- ORGANIC CHEMISTRY
CHAPERT 7 SOME BASIC
PRINCIPLES AND TECHNIQUES
VERY SHORT QUESTIONS
ANSWER
Q.1. Define the organic chemistry?
Ans. Organic chemistry is the branch of chemistry that deals
with the study of carbon-based compounds and their properties.
Q.2.What do you mean by catenation?
Ans. Catenation refers to the ability of carbon atoms to bond
with each other, forming long chains or rings, characteristic of organic
compounds.
Q.3.Define the term isomerism?
Ans. Isomerism is the phenomenon where compounds have the same
molecular formula but different arrangements of atoms, resulting in distinct
chemical and physical properties.
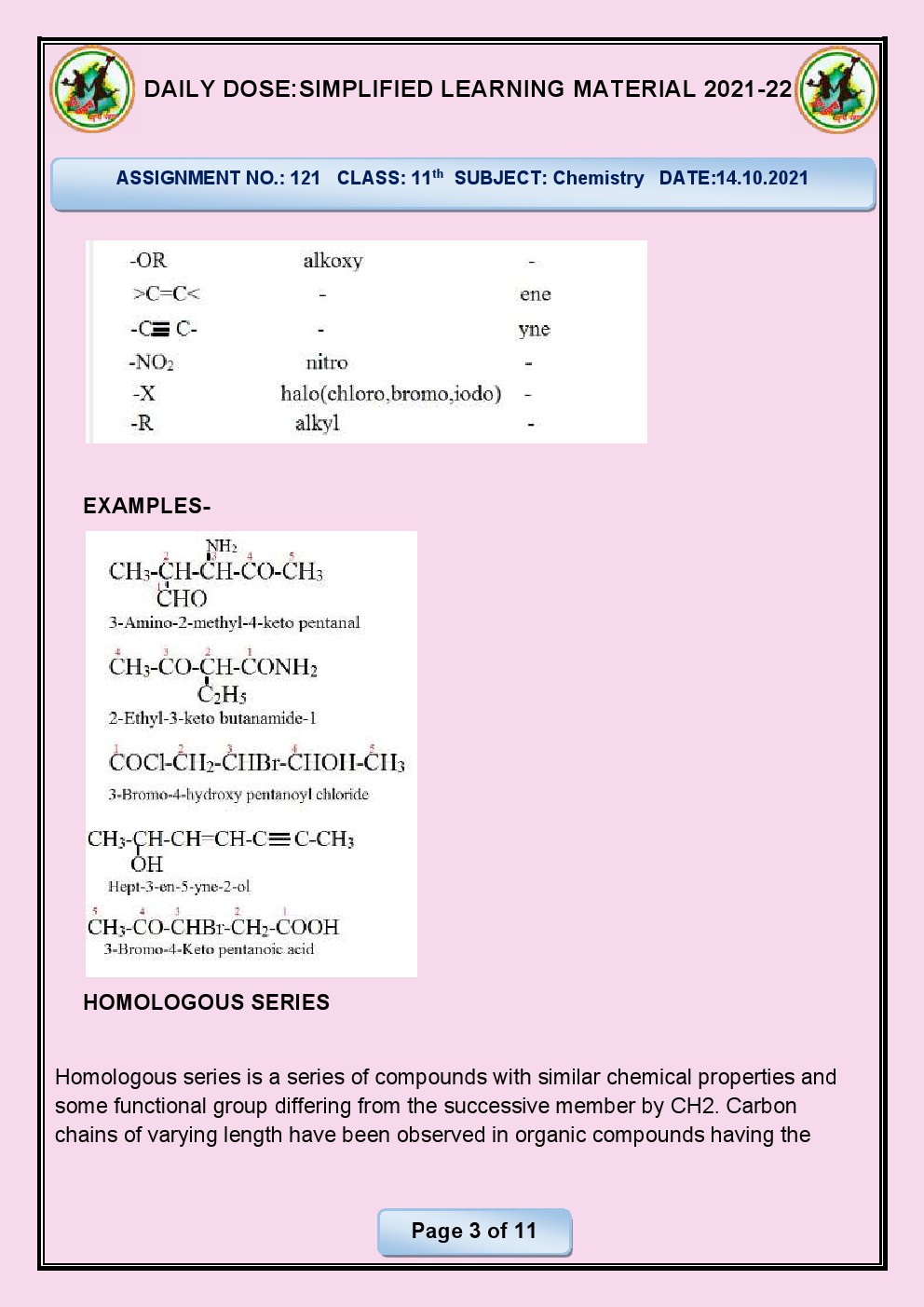
Q.4.What is meant by homologous series?
Ans. Homologous series: Group of organic compounds with
similar chemical properties and the same functional group, having a constant
difference in carbon atoms.
Q.5. Define the term functional group?
Ans. Functional group: Specific atoms or bonds in a molecule
responsible for its chemical reactivity and characteristic properties.
Q.6.What is the full expansion of
IUPAC?
Ans. IUPAC stands for the International Union of Pure and
Applied Chemistry.
Q.7.What are polyfunctional compounds?
Ans. Polyfunctional compounds: Organic compounds containing
multiple functional groups.
Q.8.What are paraffins?
Ans. Paraffins: Saturated hydrocarbons, also known as alkanes.
Q.9.What are hamocyclic or carbocyclic compounds?
Ans. Homo/Hamocyclic or Carbocyclic compounds: Organic
compounds with closed-ring structures consisting only of carbon atoms.
Q10.What is the general formula of
alkene and alkynes?
Ans. Alkene: CnH2n (where n is the number of carbon atoms).
Alkyne: CnH2n-2 (where n is
the number of carbon atoms).
Q.11.What is the IUPAC name of
neopentene?
Ans. IUPAC name of neopentene: 2,2-dimethylpropene.
Q.12.Which class do alkene and alkynes
belong to?
Ans. Alkene and alkynes belong to the class of unsaturated
hydrocarbons.
Q.13.What are aliphatic and aromatic
compounds?
Ans. Aliphatic compounds: Open-chain hydrocarbons (straight or branched).
Aromatic
compounds: Contain a cyclic ring
with alternating double bonds, like benzene.
Q.14.Name the types of structural isomerism
shown by alkanes?
Ans. Types of structural isomerism shown by alkanes: Chain
isomerism and Position isomerism.
Q.15. Name the types of structural
isomerism shown by alkanes?
Ans. Types of structural isomerism shown by alkanes: Chain
isomerism and Position isomerism.
Q.16.Name the four main types of
structural isomerism?
Ans. Four main types of structural isomerism:
Chain isomerism
Position isomerism
Functional group isomerism
Tautomerism
Q.17.What is inductive effect?
Ans. Inductive effect: The polarization of a covalent bond due to the
electronegativity difference between atoms, leading to the transmission of
electron density along a chain.
Q.18.What is electromeric effect?
Ans. Electromeric effect: The temporary displacement of
electrons in a covalent bond due to the presence of an attacking reagent,
resulting in the formation of a new temporary dipole.
Q.19.What is resonance energy?
Ans. Resonance energy: The stabilization energy gained by the delocalization of
electrons in resonance structures compared to a single contributing structure.
Q.20.What are two different types of
bond fission?
Ans. Two different types of bond fission: Homolytic bond fission (radical formation) and
Heterolytic bond fission (ion formation).
Q.21.What is heterolytic bond fission?
Ans. Heterolytic bond fission: A type of bond cleavage where
the shared pair of electrons is unequally distributed, resulting in the
formation of two ions with opposite charges.
Q.22.What is a free radical?
Ans. Free radical: A highly reactive chemical species with an
unpaired electron in its outer shell.
Q.23.What are electrophiles?
Ans. Electrophiles: Electron-deficient species that are
attracted to regions of high electron density and participate in chemical
reactions by accepting electrons.
Q.24.What are positive and neutral
electrophiles?
Ans. Positive electrophiles: Electron-deficient species with a positive charge (e.g.,
carbocations).
Neutral
electrophiles: Electron-deficient
species without a formal charge (e.g., polar molecules like HCl).
Q.25.What are nucleophiles?
Ans. Nucleophiles: Electron-rich species that are attracted to regions of
low electron density and participate in chemical reactions by donating
electrons.
Q.26.What are negative and neutral
nucleophiles?
Ans. Negative nucleophiles: Electron-rich
species with a negative charge (e.g., hydroxide ion - OH⁻).
Neutral
nucleophiles: Electron-rich species without a formal charge (e.g.,
water - H₂O, ammonia - NH₃).
Q.27.What are negative and neutral
nucleophiles?
Ans. Negative nucleophiles: Species with excess electrons and a negative charge,
seeking positively charged centers.
Neutral
nucleophiles: Species with lone
pairs of electrons that can participate in nucleophilic reactions without a
formal charge.
Q.28.Define carbonium ion?
Ans. Carbonium ion: A reactive intermediate species with a
positively charged carbon atom, also known as a carbocation, formed during
organic reactions.
Q.29. Define carbanions?
Ans. Carbanions: Reactive intermediate species with a negatively charged
carbon atom, possessing a lone pair of electrons, formed during organic
reactions.
Q.30.Which of the two electrophilic and
nuclephilic reagents would attack carbonium ion and why?
Ans. Nucleophilic reagents would attack the carbonium ion
because they are attracted to positively charged centers and donate electrons
to stabilize the positive charge on the carbon atom.
Q.31. Why are free radicals extremely
reactive?
Ans. Free radicals are extremely reactive due to the presence
of an unpaired electron in their outer shell, making them seek to pair the
electron by participating in chemical reactions.
Q.32.Give the IUPAC name of the
compounds?
Ans. I'm sorry, but you haven't provided the names of the
compounds in question. Please provide the names of the compounds, and I'll be
happy to give their IUPAC names.
Q.33.What is dry ice? Give two uses?
Ans. Dry ice is solid carbon dioxide (CO2) at temperatures
below -78.5°C.
Two uses of dry ice
are:
Cooling and preserving
perishable items during transportation.
Creating special effects in
the entertainment industry (e.g., fog effects).
Q.34.What do you mean by locant?
Ans. Locant: A term used in organic chemistry to denote the specific
location or position of a particular atom within a molecule.
Q.35. Is electromeric effect permanent?
Ans. The electromeric effect is not permanent as it occurs
only temporarily during a chemical reaction in the presence of an attacking
reagent.
Q.36.What type of solvent should be
used for crystallization?
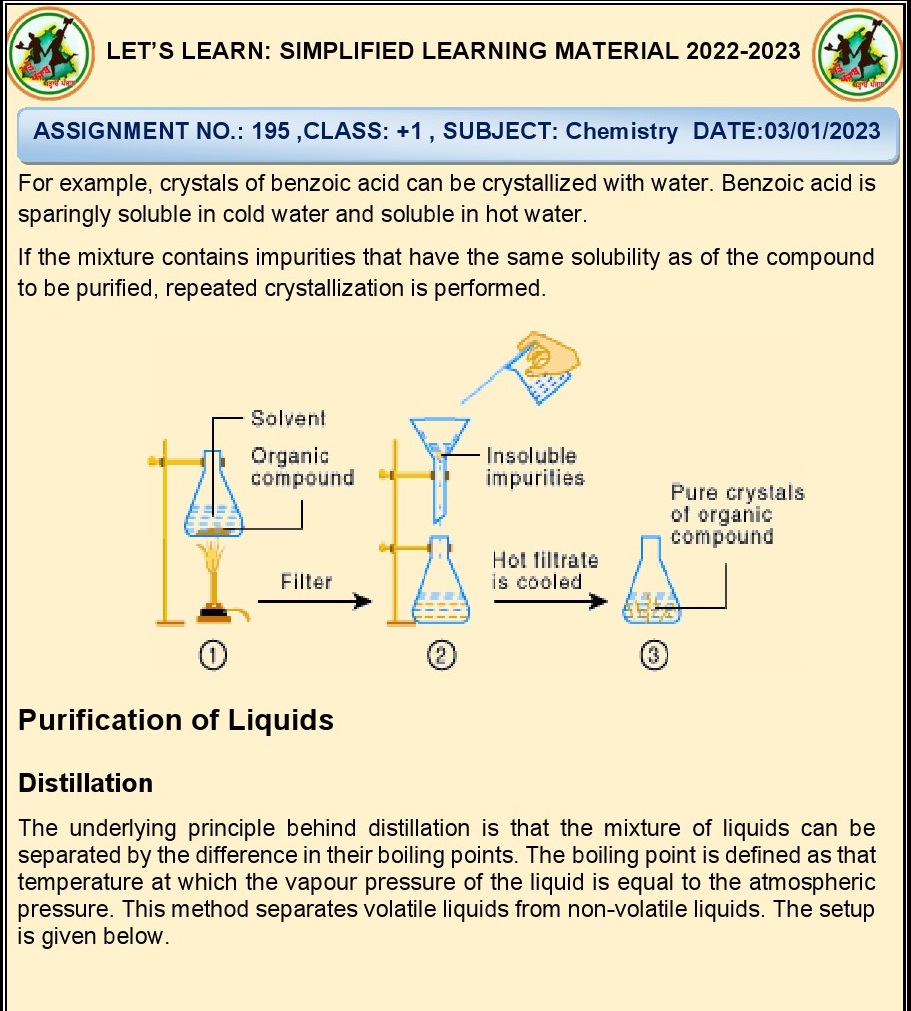
Ans. A polar solvent should be used for crystallization.
Q.37.What is seeding?
Ans. Seeding is the process of adding a small crystal of the
desired compound to a supersaturated solution to induce the growth of larger
crystals during crystallization.
Q.38. How can we separate a mixture of
benzoic acid and naphthalene?
Ans. By crystallisation.
Q.39.What is fractional
crystallisation?
Ans. Fractional crystallization: A separation technique based on the differential
solubility of components in a mixture, where crystals of the desired substance
are selectively formed and separated from the solution.
Q.40. Can urea be purified by
sublimation?
Ans. urea cannot be purified by sublimation as it decomposes
before it can sublime.
Q.41.How will you separate a mixture of
iodine and sodium chloride?
Ans. A mixture of iodine and sodium chloride can be separated
by sublimation, as iodine can sublime while sodium chloride remains as a solid.
Q.42.Which type of method will you use
to purity glyeerol?
Ans. To purify glycerol, a distillation method is commonly
used, specifically fractional distillation.
Q.43.How will you purify essential
oils?
Ans. Essential oils can be purified through a process called
steam distillation, where steam is passed through the plant material, carrying
the volatile essential oil with it. The mixture of steam and essential oil is
then condensed and separated to obtain the purified essential oil.
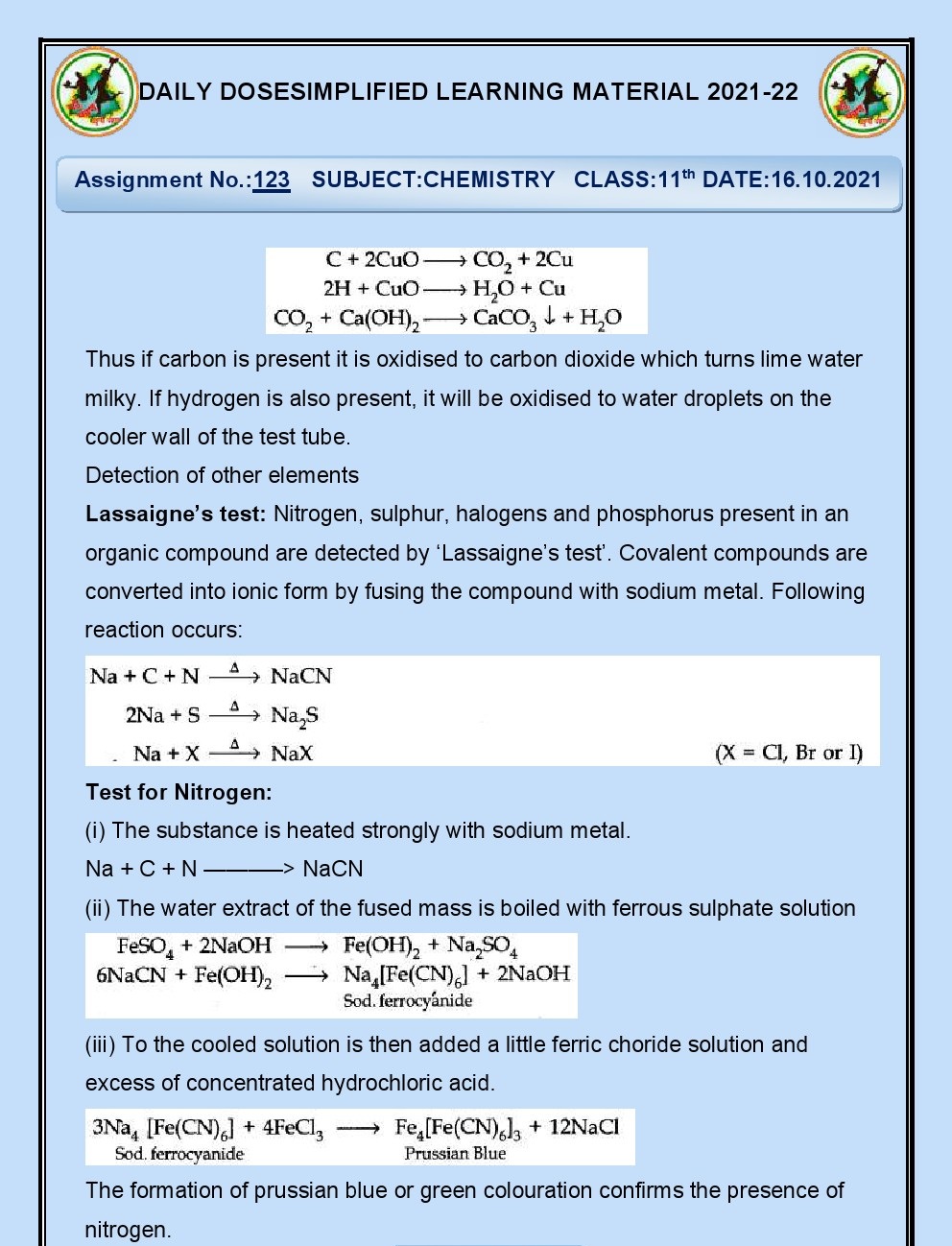
Q.44. In the lassaigne’s test for
nitrogen what is responsible for the formation of blue colour?
Ans. Ferric ferrocyanide?
Q.45.Which colour will you get in
lassaigne’s test if N and S both are present together?
Ans. Blood red colour
Q.46.Name two compounds which respond
to beilstein test but donot conatain a halogen atom?
Ans. Copper oxide and copper sulfate are two compounds that
respond positively to the Beilstein test despite not containing a halogen atom.
Q.47.Why can lithium not be used to
prepare lassaigne’s extract?
Ans. Lithium cannot be used to prepare Lassaigne's extract
because it forms soluble lithium salts, leading to false positive results for
the presence of nitrogen and sulfur.
Q.48.Is Beilstein test applicable for
the detection of fluorine?
Ans. The Beilstein test is not applicable for the detection of
fluorine because fluorine does not produce a positive test result due to its
strong oxidizing properties.
Q.49.Can we separate a mixture of
denzoic acid and camphor by suplimation?
Ans. Yes, a mixture of benzoic acid and camphor can be
separated by sublimation, as camphor can sublime while benzoic acid remains as
a solid.
Q.50.How can you separate water
insoluble phenosl from non – acidc compounds?
Ans. To separate water-insoluble phenols from non-acidic
compounds, you can use the technique of liquid-liquid extraction by reacting
the phenols with a base to form water-soluble phenolate ions that transfer to
the aqueous phase, leaving the non-acidic compounds in the organic phase.
Q.51. Why can lithium not be used to
prepare lassaigne’s extract?
Ans. Lithium cannot be used to prepare Lassaigne's extract
because it forms soluble lithium salts during the fusion process, leading to
interference and false positive results for the presence of nitrogen and
sulfur.
Q.52.Name two compounds which respond
to Beilstein test but donot contain a halogen atom?
Ans. Two compounds that respond positively to the Beilstein
test but do not contain a halogen atom are:
Copper oxide (CuO)
Copper sulfate (CuSO4)
Q.53.What is the criterion to check the
purity of organic solids?
Ans. The criterion to check the purity of organic solids is
their sharp and consistent melting point, indicating a homogeneous substance
without impurities.
Q.54.When does a liquid boil?
Ans. A liquid boils when its vapor pressure becomes equal to
the atmospheric pressure, causing bubbles of vapor to form throughout the
liquid and escape into the surroundings. This is the boiling point of the
liquid.
Q.55.When do we use hot water funnel
for filtration?
Ans. Hot water funnel filtration is used when the solid to be
filtered is soluble in hot solvent but insoluble or less soluble in cold
solvent, allowing for better separation at an elevated temperature.
Q.56. Name the apparatus used for
differential extraction?
Ans. The apparatus used for differential extraction is a
separating funnel.
Q.57. Name the compound which is formed
during carius method for estimation of phosphorus?
Ans. Silver phosphate is the compound formed during the Carius
method for the estimation of phosphorus.
Q.58.Which method is used to estimate Sulphur
and halogens quantitatively?
Ans. The Carius method is used to estimate sulfur and halogens
(chlorine, bromine, and iodine) quantitatively.
Q.59.Name the industry where fractional
distillation has been most widely used?
Ans. The petroleum industry is where fractional distillation
has been most widely used.
Q.60.What type of compounds are
generally not suitable for kjeldahlisation?
Ans. Compounds containing nitrogen in the form of nitro groups
(-NO2) are generally not suitable for Kjeldahlisation.
SHORT QESTIONS ANSWER
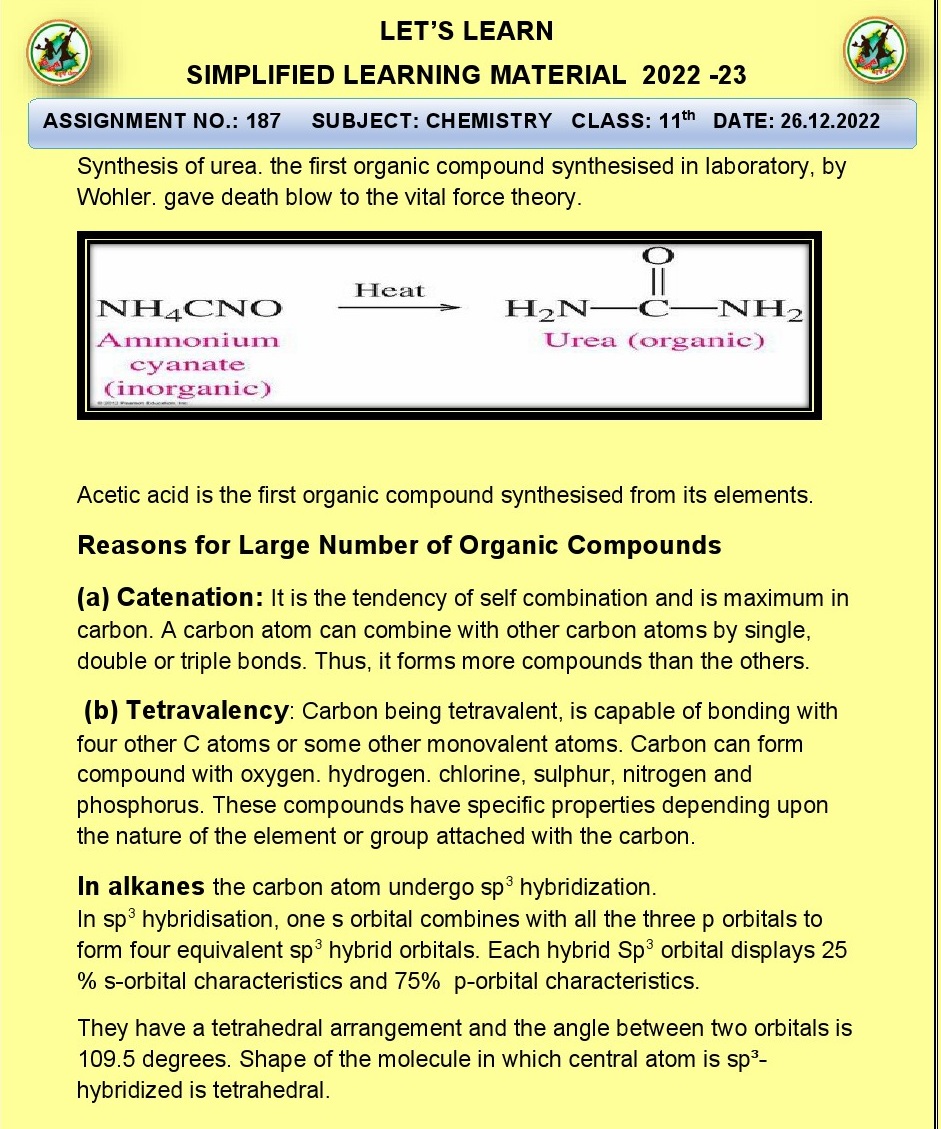
Q.1.What is vital force theory why was
it discarded?
Ans. The vital force theory was a historical concept that
proposed organic compounds could only be synthesized from living organisms due
to a vital force unique to living matter. It was discarded because Friedrich
Wöhler's synthesis of urea in 1828 proved that organic compounds could be
artificially produced from inorganic substances, disproving the theory and
paving the way for modern organic chemistry
Q.2. Explain briefly the term
catenation?
Ans. Catenation is the unique property of carbon atoms to form
strong covalent bonds with other carbon atoms, leading to the formation of long
chains, branched structures, and rings. This characteristic of carbon is
fundamental to the diversity and complexity of organic compounds.
Q.3.Catenartion is mainly shown by carbon
atom and not much in other elements why?
Ans. Catenation is mainly shown by carbon atoms and not as
much by other elements due to the following reasons:
Carbon's
electron configuration: Carbon
has four valence electrons in its outermost shell, allowing it to form strong
covalent bonds with other carbon atoms and other elements, resulting in a
variety of stable structures.
Bond
strength: Carbon-carbon bonds
are relatively strong, allowing carbon atoms to form stable chains and rings,
even at high temperatures.
Small
atomic size: Carbon has a small
atomic size, which allows for close packing and efficient overlap of orbitals,
facilitating the formation of stable covalent bonds.
Versatility: Carbon can form single, double, and triple bonds with
other carbon atoms, leading to a wide range of structures and functional groups
in organic compounds.
In contrast, other elements
may have different electron configurations, larger atomic sizes, and weaker
bond strengths, making them less suitable for extensive catenation. These
factors limit their ability to form long chains and diverse structures like
carbon.
Q.4.What are hydrocarbons name the
different types of hydrocarbons?
Ans. Hydrocarbons are organic compounds consisting of hydrogen
and carbon atoms. The main types of hydrocarbons are:
Alkanes: They have single covalent bonds between carbon atoms.
General formula: CnH2n+2.
Alkenes: They have at least one double covalent bond between
carbon atoms. General formula: CnH2n.
Alkynes: They have at least one triple covalent bond between
carbon atoms. General formula: CnH2n-2.
Aromatic
hydrocarbons: They contain a cyclic
ring structure with alternating single and double bonds, such as benzene.
Hydrocarbons are the
building blocks of many organic compounds and are essential in various
industrial processes and natural occurrences
Q.5.What are primary secondary tertiary
and quarternary carbon atoms?
Ans. Primary, secondary, tertiary, and quaternary carbon atoms
are classifications based on the number of carbon atoms bonded to a given carbon
atom in an organic compound.
Primary
carbon (1°): A carbon atom
directly bonded to one other carbon atom.
Secondary
carbon (2°): A carbon atom
directly bonded to two other carbon atoms.
Tertiary
carbon (3°): A carbon atom
directly bonded to three other carbon atoms.
Quaternary
carbon (4°): A carbon atom
directly bonded to four other carbon atoms.
These classifications help
describe the carbon's chemical environment and reactivity in a molecule and are
important in understanding the reactions and behavior of organic compounds.
Q.6. Write main characteristics of a
homologous series?
Ans. Main characteristics of a homologous series are:
Same
functional group: All
compounds in a homologous series have the same functional group, which imparts
similar chemical properties.
Gradation
in physical properties: There
is a gradual increase in molecular size and mass as one moves from one member
of the series to the next, resulting in a predictable pattern of physical
properties (e.g., boiling points, melting points).
Same
general formula: Members
of a homologous series follow a general formula, showing a constant difference
in the number of carbon atoms and hydrogen atoms in their molecular structure.
Similar
chemical reactivity: Due
to the presence of the same functional group, compounds in a homologous series
exhibit similar chemical reactivity and undergo analogous chemical reactions.
Trend
in physical and chemical properties: As the number of carbon atoms increases in a homologous
series, there is often a trend in physical and chemical properties, making it
easier to predict the behavior of new members of the series.
The concept of homologous
series is fundamental in organic chemistry as it helps in understanding the
relationships between different organic compounds and simplifies the study of a
wide range of related compounds.
Q.7.What do you mean by Tautomerism?
Give examples also?
Ans. Tautomerism is a type of isomerism where a molecule can
exist in two different forms (tautomers) that rapidly interconvert by a
reversible chemical reaction. The tautomers have the same molecular formula but
differ in the placement of a hydrogen atom and the double bond.
Example
1: Keto-enol tautomerism
in ketones and enols:
Ketone
form: Acetone (CH3-CO-CH3)
Enol
form: Prop-2-en-1-ol
(CH2=CH-C(OH)-CH3)
Example
2: Aldehyde-ketone
tautomerism in aldehydes and ketones:
Aldehyde
form: Propanal
(CH3-CH2-CHO)
Ketone
form: Propanone
(CH3-CO-CH3)
In tautomerism, the shift of
a proton and rearrangement of double bonds lead to the existence of two
isomeric forms that are in dynamic equilibrium. Tautomerism is crucial in
biochemistry and organic reactions, and it plays a significant role in the
behavior of certain compounds.
Q.8. Between formic acid and acetic
acid which is stronger acid and why?
Ans. Acetic acid (CH3COOH) is a stronger acid than formic acid
(HCOOH).
The strength of an acid is
determined by its ability to donate a proton (H+) in a solution. In acetic
acid, the presence of an electron-withdrawing group (the carbonyl group -COOH)
adjacent to the carboxylic acid functional group (-COOH) stabilizes the
conjugate base (CH3COO-) formed after losing a proton. This stabilization
results from the resonance effect, where electron delocalization occurs in the
conjugate base.
In contrast, formic acid
lacks this electron-withdrawing group, so the stabilization of its conjugate
base (HCOO-) is weaker compared to acetic acid. Consequently, acetic acid is
more acidic than formic acid because it can more readily donate a proton in a
solution.
Q.9.What is hyper conjugation effect Give
an example also?
Ans. Hyperconjugation is a stabilizing interaction in organic
chemistry where the overlap of a sigma (σ) bond or a lone pair of electrons on
an adjacent carbon atom with an empty or partially filled orbital of a
neighboring atom results in the delocalization of electrons.
Example: Hyperconjugation in the stability of alkyl carbocations.
In the case of an alkyl
carbocation, such as a tertiary carbocation (R3C+), the positive charge is
localized on the central carbon atom. However, neighboring carbon-hydrogen
(C-H) bonds can interact with the vacant p-orbital on the positively charged
carbon, leading to hyperconjugation. This interaction results in the
delocalization of electron density, reducing the charge concentration on the
carbon atom and stabilizing the carbocation.
For example, in the
reaction:
CH3-CH2-CH2+ → CH3+ + CH2=CH2
The stability of the
tertiary carbocation (CH3-CH2-CH2+) is increased due to the hyperconjugation
effect, making it more stable than a primary or secondary carbocation.
Q.10.What are the consequences of hyper
conjugation effect? Explain?
Ans. The consequences of the hyperconjugation effect in organic
chemistry are as follows:
Stabilization
of carbocations: Hyperconjugation
stabilizes carbocations by delocalizing electron density from adjacent sigma
(σ) bonds or lone pairs of electrons onto the positively charged carbon atom.
This reduces the charge concentration on the carbon, making the carbocation
more stable.
Influence
on acidity: Hyperconjugation can
also affect the acidity of certain compounds. For example, in alkyl-substituted
carboxylic acids, hyperconjugation can stabilize the negative charge on the
carboxylate ion, making the carboxylic acid more acidic compared to simple
acetic acid.
Influence
on bond lengths and bond angles: Hyperconjugation can influence the bond lengths and bond
angles in molecules. The delocalization of electron density can lead to partial
double bond character in sigma bonds, resulting in shorter bond lengths and
altered bond angles.
Impact
on reactivity: The presence of hyperconjugation
can impact the reactivity of certain compounds. For example, it can affect the
stability of radicals and transition states in organic reactions, influencing
reaction pathways.
Overall, the
hyperconjugation effect plays a significant role in determining the stability,
reactivity, and properties of various organic compounds. It is an essential
concept in understanding the behavior of organic molecules and their reactions.
Q.11.What are rearrangement reactions give
one example also?
Ans. Rearrangement reactions are chemical reactions in which
the atoms or groups within a molecule are rearranged to form a different
structural isomer of the original compound. These reactions often occur through
the migration of atoms or groups within the molecule.
Example of a
rearrangement reaction:
Hydride shift in the
Wagner-Meerwein rearrangement:
CH3
\
CH3-CH2-CH2-C+H2
\
CH3
In this example, the
Wagner-Meerwein rearrangement involves the migration of a hydride ion (H-) from
the methyl group on the left to the positively charged carbon (carbocation) in
the center. This rearrangement leads to the formation of a more stable tertiary
carbocation. Subsequently, a nucleophile can attack the carbocation to form a
new product with a different carbon skeleton.
Q.12. Is cyclohexyl amine is more basic
than aniline Give reason?
Ans. Cyclohexylamine is more basic than aniline.
Reason: In aniline, the lone pair of electrons on the nitrogen
atom is partially delocalized into the benzene ring through resonance, reducing
its availability for donation to a proton (H+). This partial delocalization decreases
the basicity of aniline.
On the other hand, in
cyclohexylamine, the lone pair of electrons on the nitrogen atom is not
delocalized into any aromatic system, making it more available for donation to
a proton. As a result, cyclohexylamine is more basic than aniline.
Q.13. Give two methods of generation of
carbocation?
Ans. Two methods of generating carbocations are:
Protonation of alkenes: In
this method, an alkene reacts with a strong acid, such as sulfuric acid (H2SO4)
or hydrochloric acid (HCl), to form a carbocation as an intermediate in the
reaction.
Example:
CH2=CH2 + H2SO4 → CH3-CH2^+ + HSO4^-
Tertiary alcohol
dehydration: When a tertiary alcohol is subjected to dehydration in the
presence of a strong acid catalyst, it loses a water molecule to form a
carbocation.
Example:
(CH3)3C-OH + H2SO4 → (CH3)3C^+ + H2O
These methods create
carbocations as short-lived intermediates, which are highly reactive and
participate in various organic reactions.
Q.14.What are carbanions how are these
generated?
Ans. Carbanions are chemical species that possess a negatively
charged carbon atom with three lone pairs of electrons. They are strong
nucleophiles and can donate a pair of electrons to other atoms during chemical
reactions.
Carbanions can be
generated through various methods:
Deprotonation
of carbon acids: When
a compound contains an acidic hydrogen directly attached to a carbon atom, it
can be removed by a strong base (e.g., hydroxide ion - OH⁻) to form a
carbanion.
Example:
CH3-CH2-C≡C-H + NaNH2 → CH3-CH2-C≡C^- + NH3 + NaH
Nucleophilic
substitution: In certain reactions,
a nucleophile can attack an electron-deficient carbon, leading to the formation
of a carbanion intermediate.
Example:
CH3-Br + :OH⁻ → CH3^- + :BrOH
Reduction
of carbocations: Carbocations
(positively charged carbon atoms) can be reduced to form carbanions by the
addition of electrons.
Example:
(CH3)3C^+ + 2e^- → (CH3)3C^-
Carbanions are essential
intermediates in organic reactions and play a crucial role in nucleophilic
substitution and other reaction mechanisms.
Q.15.What is resonance give suitable
examples?
Ans. Resonance is a concept in chemistry that describes the
delocalization of electrons in a molecule or ion over multiple atoms or bonds.
It occurs when a molecule can be represented by two or more Lewis structures
(resonance structures) that differ only in the arrangement of electrons while
keeping the same atomic positions.
Examples of resonance:
Benzene: Benzene (C6H6) is a classic example of resonance. It can
be represented by two resonance structures, each having alternating single and
double bonds between carbon atoms.
H H
\ /
C==C
/ \
H H
Nitrate
ion: The nitrate ion
(NO3^-) can be represented by two resonance structures.
O O
/ \
/ \
N O-
N O-
\ /
\ /
O O
Resonance structures help
explain the stability and reactivity of certain molecules and ions. The actual
electronic structure is a hybrid of the resonance forms, with electron density
being shared across the entire molecule, making it more stable.
Q.16.What are various reactions
intermediates how are they formed?
Ans. Various reaction intermediates in organic chemistry
include:
Carbocations: Carbocations are positively charged carbon atoms that are
formed during reactions through heterolytic bond cleavage, where one atom takes
both electrons from a bond, leaving the other atom with a positive charge.
Carbanions: Carbanions are negatively charged carbon atoms with three
lone pairs of electrons, generated by the deprotonation of carbon acids or
nucleophilic attack on electron-deficient carbons.
Free
radicals: Free radicals are
highly reactive species with unpaired electrons, formed by homolytic bond
cleavage, where each atom retains one electron from the bond.
Carbenes: Carbenes are neutral species with a divalent carbon atom,
formed by the elimination of a small molecule (e.g., diazo compound) from a
precursor.
Nitrenes: Nitrenes are nitrogen analogs of carbenes, neutral
species with a divalent nitrogen atom, formed by the elimination of a small
molecule (e.g., azide) from a precursor.
These reaction intermediates
play critical roles in various organic reactions and often determine the
selectivity and mechanism of the reactions they are involved in.
Q.17.What are nucleophilic reactions and
nucleophilic addition reactions give examples also?
Ans. Nucleophilic reactions involve the participation of
nucleophiles, which are electron-rich species that seek positively charged
centers (such as electrophiles) to donate a pair of electrons and form new chemical
bonds.
Nucleophilic addition
reactions are a specific type of nucleophilic reaction where a nucleophile adds
to a molecule, leading to the formation of a new product with an additional
group or atom.
Examples of nucleophilic
reactions:
Nucleophilic addition
to carbonyl compounds:
CH3-C=O
+ :NH3 → CH3-C(-NH2)-OH
(Acetaldehyde + Ammonia → Ethanolamine)
Nucleophilic addition to
alkenes (hydrogen halide addition):
CH2=CH2 + HBr → CH3-CH2-Br
(Ethene + Hydrogen bromide → Bromoethane)
Nucleophilic substitution
(SN1 and SN2 reactions):
SN1: R-LG → R^+ + LG^- → R-Nu + LG^- (where R is an alkyl
group, LG is a leaving group, and Nu is a nucleophile)
SN2: Nu^- + R-LG → R-Nu + LG^- (where R is an alkyl group, LG
is a leaving group, and Nu is a nucleophile)
In nucleophilic addition
reactions, the nucleophile attacks an electrophilic center, resulting in the
addition of the nucleophile to the substrate. These reactions are crucial in
organic synthesis and the formation of new carbon-carbon and carbon-heteroatom
bonds.
Q.18. How will you compare inductive
effect with Electrometic effect?
Ans. Inductive effect and electromeric effect are both
electronic effects that influence the distribution of electrons in a molecule,
but they operate through different mechanisms:
Inductive effect:
It involves the polarization
of sigma (σ) bonds in a molecule due to the electronegativity difference
between atoms.
It operates through the
sigma (σ) bonds and affects the electron density along the sigma bond.
It is a permanent effect, as
the polarized bond retains its polarization even in the absence of a reaction
or other influences.
The inductive effect is
transmitted through sigma bonds and is usually weaker over longer distances.
Electromeric effect (also
called mesomeric or resonance effect):
It involves the
delocalization of pi (π) electrons in a molecule through resonance structures,
particularly in conjugated systems (e.g., double bonds and aromatic rings).
It operates through the pi
(π) bonds and affects the electron density in the entire conjugated system.
It is a temporary effect, as
it arises due to the rapid interconversion of resonance structures during a
chemical reaction or in response to external influences.
The electromeric effect can
be transmitted through multiple bonds and can be more influential over longer
distances due to resonance.
In summary, the inductive
effect is a permanent, localized effect along sigma bonds, while the
electromeric effect is a temporary, delocalized effect involving the resonance
structures in a conjugated system. Both effects play essential roles in
determining the chemical properties and reactivity of organic compounds.
Q.19. Write difference between
Tautomerism and Resonance?
Ans. Tautomerism and resonance are two different concepts in
organic chemistry related to the distribution of electrons in molecules:
Tautomerism:
Tautomerism involves the
isomerization of a molecule into another structural isomer, known as a
tautomer, through the movement of atoms or groups within the molecule.
The tautomers are in dynamic
equilibrium, meaning they rapidly interconvert through a reversible chemical
reaction.
Tautomerism results in
different chemical compounds with distinct physical and chemical properties.
It is a phenomenon observed
in certain functional groups like keto-enol tautomerism in ketones and enols.
Resonance:
Resonance involves the
delocalization of electrons within a molecule through the formation of multiple
resonance structures.
Resonance structures are
hypothetical representations of the molecule, where the actual electronic
structure is considered a hybrid of all resonance contributors.
Resonance stabilizes the
molecule by distributing electron density over a larger area, making it more
stable than any individual resonance structure.
It is commonly observed in
conjugated systems, such as in benzene and other aromatic compounds.
In summary, tautomerism is
the interconversion of structural isomers through atom or group movement,
leading to different compounds in dynamic equilibrium. Resonance, on the other
hand, involves electron delocalization and stabilization through the formation
of multiple resonance structures within a molecule.
Q.20. Give brief account of kjeldahl’s
method for estimation of nitrogen?
Ans. Kjeldahl's method is a
widely used analytical technique for the estimation of nitrogen content in
organic compounds. It is named after its inventor, Johan Kjeldahl, a Danish
chemist.
The Kjeldahl method
involves the following steps:
Digestion: The sample containing the nitrogen compound is digested
with concentrated sulfuric acid (H2SO4) in the presence of a catalyst (e.g.,
copper sulfate or selenium). During digestion, organic nitrogen compounds are
converted into ammonium sulfate (NH4)2SO4.
Distillation: After digestion, the mixture is diluted with water and
heated to release ammonia gas (NH3). The ammonia is then collected in an acidic
solution (usually boric acid) through steam distillation.
Titration: The ammonia collected in the acidic solution is then
titrated with a standard solution of a strong base, such as sodium hydroxide
(NaOH). The amount of ammonia reacted in the titration allows the calculation
of the nitrogen content in the original sample.
The Kjeldahl method is
widely used in various industries, including food and agriculture, to determine
the protein content in food and the nitrogen content in soil and fertilizers.
It is a reliable and precise method for nitrogen estimation.
Q.21. How is estimation of carbon and
hydrogen carried out by Liebig’s method?
Ans. Liebig's method, also known as the combustion method, is
used for the estimation of carbon and hydrogen in organic compounds. It
involves the complete combustion of a known mass of the organic compound in a
combustion tube, followed by the absorption of the resulting combustion
products in specific absorbing solutions.
The steps of Liebig's
method are as follows:
Weighing
the sample: A known mass of the
organic compound is accurately weighed.
Combustion: The weighed sample is mixed with copper oxide (CuO) as an
oxidizing agent and placed in a combustion tube. The tube is then heated to
high temperatures, causing the complete combustion of the organic compound.
Absorption
of combustion products: The
combustion products, which mainly consist of carbon dioxide (CO2) and water
(H2O), are passed through specific absorbing solutions. Carbon dioxide is
absorbed in a solution of potassium hydroxide (KOH), while water is absorbed in
a tube containing calcium chloride (CaCl2).
Weighing
the absorbent tubes: After
the combustion products are absorbed, the tubes containing potassium hydroxide
and calcium chloride are weighed to determine the increase in their mass due to
the absorption of carbon dioxide and water, respectively.
Calculations: The increase in mass of the potassium hydroxide tube
corresponds to the amount of carbon in the sample, and the increase in mass of
the calcium chloride tube corresponds to the amount of hydrogen in the sample.
By comparing the mass increase with the initial mass of the sample, the percentage
of carbon and hydrogen in the compound can be calculated.
Liebig's method is a classic
and widely used technique for the estimation of carbon and hydrogen in organic
compounds.
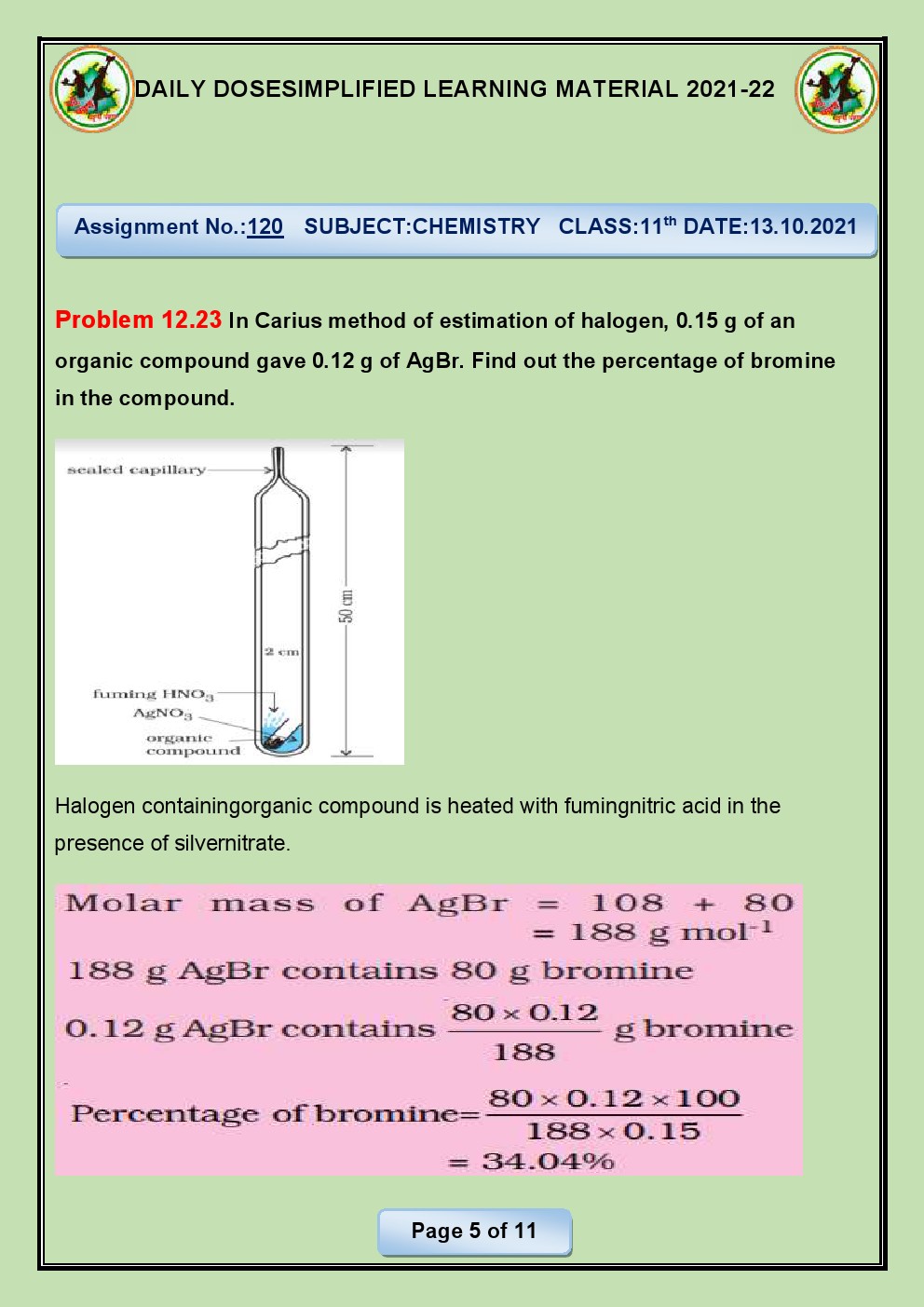
Q.22. How is Sulphur estimated
quantitatively in an organic compound?
Ans. Sulfur can be quantitatively estimated in an organic
compound using the Carius method. The Carius method involves the oxidation of
organic sulfur compounds to sulfuric acid (H2SO4) in the presence of fuming
nitric acid (HNO3) at high temperatures. The sulfuric acid formed is then
precipitated as barium sulfate (BaSO4) and weighed to determine the amount of
sulfur present in the original sample.
The steps of the
Carius method are as follows:
Weighing
the sample: A known mass of the
organic compound containing sulfur is accurately weighed.
Digestion: The weighed sample is placed in a sealed tube with fuming
nitric acid (concentrated nitric acid with dissolved nitrogen dioxide). The
tube is then heated at high temperatures (around 300-400°C) for a few hours. During
this process, sulfur in the organic compound is oxidized to sulfuric acid by
the fuming nitric acid.
Precipitation:
After digestion, the tube is allowed to
cool, and the contents are transferred to a beaker. Barium chloride solution
(BaCl2) is added to the beaker, resulting in the formation of a white
precipitate of barium sulfate (BaSO4) due to the reaction of barium chloride
with sulfuric acid.
Filtration
and weighing: The barium sulfate
precipitate is filtered, washed, dried, and weighed. The weight of the barium
sulfate is used to calculate the percentage of sulfur in the original sample.
The Carius method provides a
quantitative estimation of sulfur in organic compounds and is widely used in
analytical chemistry for sulfur determination.