7- EQUILIBRIUR
CHAPTER 10 EQUILIBRIUM
VERY QUESTIONS ANSWER
Q.1.What is meant by equilibrium?
Ans. Equilibrium is a state of balance or stability in a
system where opposing forces or processes are in perfect balance, and there is
no net change occurring.
Q.2.Define reversible process?
Ans. A reversible process is a thermodynamic process that can
be undone without leaving any trace, as it occurs slowly and in perfect
equilibrium at each step.
Q.3.Do reversible reactions go to
completion?
Ans. No.
Q.4.What is physical equilibrium? give
an example.
Ans. Physical equilibrium is a state where all forces acting
on an object balance out, resulting in no net change in the object's motion;
for example, a book resting on a flat table.
Q.5.Name three factors by which
equilibrium state of reaction is disturbed.
Ans. Temperature change, pressure change, and concentration
change.
Q.6. what is meant by which equilibrium
is dynamic in nature”?
Ans. The phrase "equilibrium is dynamic in nature"
means that the forward and reverse reactions continue to occur at the same
rate, resulting in no net change in concentrations.
Q.7.Can equilibrium be achieved in open
container if products can escape?
Ans. Yes.
Q.8.what are the conditions for getting
maximum yield of so3 by contact process?
Ans. The conditions for getting the maximum yield of SO3 in
the contact process are using a low temperature, high pressure, and a suitable
catalyst (vanadium pentoxide).
Q.9.Can a catalyst disturb the
equilibrium in a reaction?
Ans. No, a catalyst does not disturb the equilibrium in a
reaction.
Q.10.State the law of mass action?
Ans. The law of mass action states that the rate of a chemical
reaction is directly proportional to the product of the concentrations of the
reactants, each raised to the power of its stoichiometric coefficient.
Q.11.Define equilibrium constant and
state law of chemical equilibrium?
Ans. Equilibrium constant: Numerical value representing the
ratio of product concentrations to reactant concentrations at equilibrium.
Q.12.Stae Le-chatelier’s principle?
Ans. Le Chatelier's principle: When a system at equilibrium is
subjected to an external stress, it will adjust itself to partially counteract
the effect of the stress and restore a new equilibrium state.
Q.13.What is active mass?
Ans. Active mass: The concentration of a species in a reaction, usually expressed
in terms of molarity (moles per liter).
Q.14.Under what condition a reversible
process becomes irreversible?
Ans. A reversible process becomes irreversible when there is a
large difference between the system's initial state and the surroundings, leading
to significant irreversibilities and energy dissipation.
Q.15.Name the principle involved behind
skating on ice?
Ans. The principle involved behind skating on ice is reducing
friction through the formation of a thin layer of water from the pressure exerted
by the skates, allowing for smooth gliding.
Q.16.At what temperature the solid and liquid
are in equilibrium under I am pressure?
Ans. At melting point or
freezing point?
Q.17.What does happen when pressure is
applied on ice?
Ans. When pressure is applied to ice, it can melt and form a
thin layer of water, reducing friction and allowing for easier movement.
Q.18.What does the value of equilibrium
constant depend upon?
Ans. The value of the equilibrium constant depends on the
specific chemical reaction and its stoichiometry at a given temperature.
Q.19.What is the thermodynamic
criterion for the state of equilibrium?
Ans. The thermodynamic criterion for the state of equilibrium
is that the Gibbs free energy change (ΔG) of the system is zero.
Q.20.What is the value of ΔG
at equilibrium?
Ans. ΔG=0.
Q.21.What do you mean by ionic
equilibrium?
Ans. Ionic equilibrium refers to the balance between the
ionized and unionized forms of electrolytes in a solution.
Q.22.State Ostwald dilution law?
Ans. Ostwald dilution law: The square root of the ratio of the degree of
dissociation to the initial concentration of a weak electrolyte is directly
proportional to the dilution of the solution.
Q.23.Define electrolyte and
non-electrolyte?
Ans. Electrolyte: A substance that produces ions when dissolved in a solvent,
conducting electricity.
Non-electrolyte: A substance that does not produce ions when dissolved in
a solvent, not conducting electricity.
Q.24.Define degree of dissociation?
Ans. Degree of dissociation: The fraction of molecules that
dissociate into ions in a solution of a weak electrolyte.
Q.25.Define PH?
Ans. pH: A measure of the acidity or alkalinity of a solution,
representing the negative logarithm of the hydrogen ion concentration.
Q.26.Name a substance which can act
both as Bronsted acid as base?
Ans. Water (H2O) can act as both a Bronsted acid and a
Bronsted base.
Q.27.What is meant by ionic product of
water?
Ans. The ionic product of water is the product of the
concentrations of hydrogen ions (H+) and hydroxide ions (OH-) in pure water at
a given temperature, denoted as Kw.
Q.28.What do you mean by common ion
effect?
Ans. Common ion effect refers to the suppression of the
ionization of a weak electrolyte by the presence of a strong electrolyte
containing a common ion.
Q.29.What is the PH of human blood?
Ans. The pH of human blood is approximately 7.4.
Q.30.What is POH?
Ans. pOH is the negative logarithm of the hydroxide ion
concentration and is used to express the basicity of a solution.
Q.31.What is the limitation of PH
scale?
Ans. The pH scale is limited to measuring the acidity or
basicity of aqueous solutions and does not provide information about the
strength or concentration of acids and bases.
Q.32.What is an indicator?
Ans. An indicator is a substance that changes color in
response to changes in pH, used to determine the acidity or basicity of a
solution.
Q.33.What do you mean by Degree of
hydrolysis?
Ans. Degree of hydrolysis refers to the extent of a substance
that has undergone hydrolysis, typically expressed as a percentage.
Q.34. Give limitations of Lewis
concept?
Ans. Lewis concept does not explain the acid-base behavior in
non-aqueous solvents and fails to predict the strengths of certain acids and
bases accurately.
Q.35.What is PH scale?
Ans. The pH scale is a numerical scale used to specify the
acidity or basicity of an aqueous solution, ranging from 0 (strongly acidic) to
14 (strongly basic), with 7 being neutral.
Q.36.Who introduced PH scale?
Ans. The concept of the pH scale was introduced by the Danish
chemist Søren Peder Lauritz Sorensen in 1909.
Q.37.Define buffer solution give an
example?
Ans. Buffer solution resists changes in pH upon addition of
small amounts of acid or base; Example: Acetic acid-sodium acetate buffer.
Q.38.Give two characteristics of s
buffer solution?
Ans. Two characteristics of a buffer solution: It maintains a
nearly constant pH upon addition of small amounts of acid or base and contains
a weak acid/base and its conjugate salt.
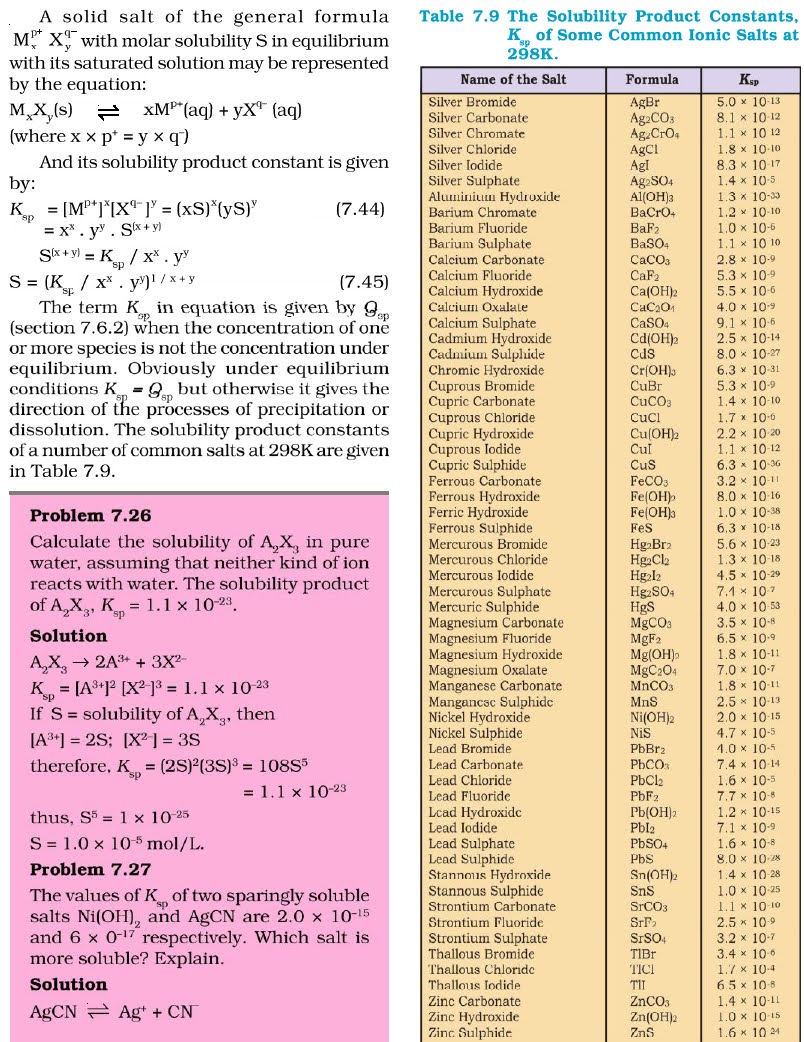
Q.39.What do you mean by solubility
product?
Ans. Solubility product is the equilibrium constant for the
dissolution of a sparingly soluble salt in water, representing the product of
ion concentrations at saturation.
Q.40.What is buffer capacity?
Ans. Buffer capacity is the amount of acid or base that a
buffer solution can neutralize while maintaining its pH within a certain range.
Q.41.What are acidic buffers?
Ans. Acidic buffers are buffer solutions with pH values less
than 7, containing a weak acid and its conjugate base.
Q.42.What is basic buffer?
Ans. Basic buffers are buffer solutions with pH values greater
than 7, containing a weak base and its conjugate acid.
Q.43.Name an important buffer in
biology?
Ans. Hemoglobin is an important buffer in biology.
SHORT QUESTIONS ANSWER
Q.1.State and explain Henry‘s law?
Ans. Henry's law states that at a constant temperature, the
amount of gas dissolved in a liquid is directly proportional to the partial
pressure of the gas above the liquid.
Explanation: When a gas is in contact with a liquid, some of its
molecules will dissolve into the liquid phase until equilibrium is reached. The
rate at which the gas dissolves is directly proportional to the partial
pressure of the gas above the liquid. This means that if the partial pressure
of the gas increases, more gas molecules will dissolve into the liquid, and if
the partial pressure decreases, some of the dissolved gas will come out of the
liquid and return to the gaseous phase. Henry's law is essential in
understanding gas solubility in various applications, such as in the
dissolution of gases in beverages, in gas exchange between blood and lungs, and
in environmental processes like the absorption of gases in water bodies.
Q.2.Explain why can pure liquids and
solids be ignored while writing the value of equilibrium?
Ans. Pure liquids and solids are usually ignored while writing
the value of an equilibrium constant because their concentrations remain
constant in a closed system, and they do not affect the equilibrium position.
In a chemical reaction
involving pure liquids or solids, their concentrations are constant as long as
the temperature and pressure remain constant. Therefore, their concentrations
do not change during the course of the reaction, and they do not appear in the
expression for the equilibrium constant.
For example, consider
the reaction:
�
(
�
)
+
�
(
�
)
⇌
�
(
�
)
A(s)+B(g)⇌C(l)
The equilibrium expression
for this reaction would be:
�
=
[
�
]
[
�
]
K=
[B]
[C]
Since the concentration of
the pure solid A does not change, it is not included in the equilibrium
expression. Similarly, the concentration of the pure liquid C does not change,
so it is also not included in the equilibrium expression.
Ignoring the pure liquids
and solids in the equilibrium expression simplifies the expression and makes it
easier to calculate the equilibrium constant based on the concentrations of
only the reacting species, which can vary during the course of the reaction.
Q.3.What do you mean by homogeneous
equilibria and heterogeneous equilibria?
Ans. Homogeneous equilibria occur when all the reactants and
products in a chemical reaction are in the same phase (e.g., all gases, all
liquids, or all aqueous solutions).
Heterogeneous equilibria, on
the other hand, occur when the reactants and products are in different phases
(e.g., a gas reacting with a solid, a liquid reacting with a gas, etc.).
Q.4.What is the law of chemical
equilibrium?
Ans. The law of chemical equilibrium is also known as the law
of mass action. It states that the rate of a chemical reaction at equilibrium
is proportional to the product of the concentrations (or partial pressures) of
the reactants, each raised to the power of their respective stoichiometric
coefficients in the balanced chemical equation. This law is expressed by the
equilibrium constant (Kc for concentrations and Kp for partial pressures). At
equilibrium, the ratio of the concentrations (or partial pressures) of products
to reactants remains constant, and this constant value is the equilibrium
constant.
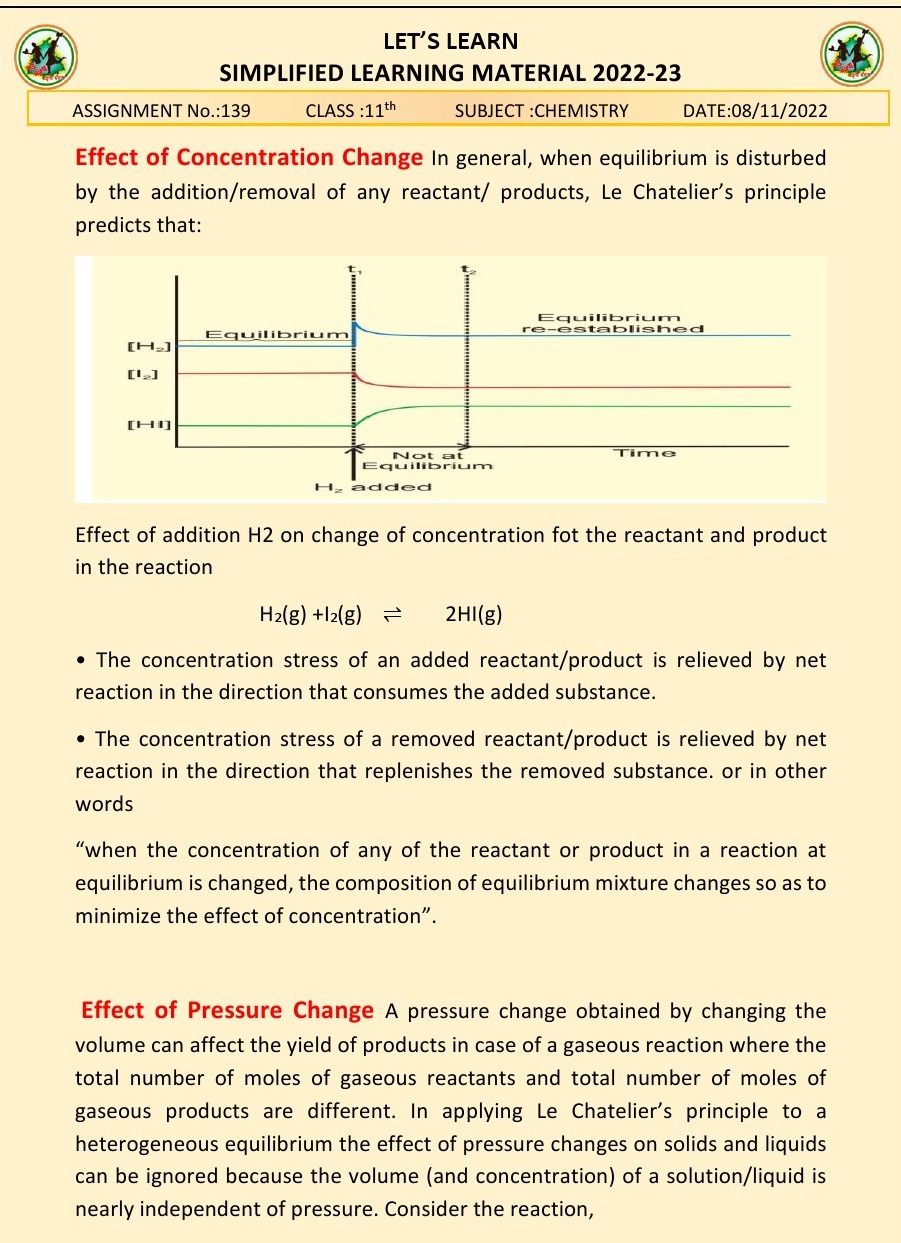
Q.5.What do you mean be Le-chateeller’s
principle?
Ans. Le Chatelier's principle states that when a system at
equilibrium is subjected to a change in temperature, pressure, or concentration
of reactants or products, the system will shift its position of equilibrium to
counteract that change and establish a new equilibrium. In other words, if a
system is disturbed from its equilibrium state, it will respond in a way that
tends to undo the disturbance and restore equilibrium. This principle helps
predict how changes in conditions will affect the position of equilibrium in a
chemical reaction.
Q.6.Why do sweets cause tooth decay?
Ans. Sweets cause tooth decay because the bacteria in our
mouth feed on the sugars and produce acids as byproducts. These acids can erode
the enamel, the protective outer layer of the teeth, leading to cavities or
tooth decay. Regular consumption of sugary foods without proper oral hygiene
can increase the risk of tooth decay and other dental issues.
Q.7.How does oxygen transport by
haemoglobin in blood obeying Le-chatelier‘s principle?
Ans. The oxygen transport by hemoglobin in blood obeys Le
Chatelier's principle through its ability to respond to changes in partial
pressure of oxygen (pO2) in the surrounding environment.
When blood reaches the
lungs, where the pO2 is relatively high, hemoglobin binds to oxygen to form
oxyhemoglobin, according to the following reaction:
�
�
+
4
�
2
⇌
�
�
(
�
2
)
4
Hb+4O2⇌Hb(O2)4
This reaction is favored in
the lungs due to the high pO2, and hemoglobin readily binds to oxygen to form
oxyhemoglobin.
Conversely, when blood
reaches the body tissues, where the pO2 is relatively low (due to oxygen being
used by cells), the equilibrium shifts to the left, and oxyhemoglobin releases
oxygen to the tissues for cellular respiration:
�
�
(
�
2
)
4
⇌
�
�
+
4
�
2
Hb(O2)4⇌Hb+4O2
By shifting the equilibrium
in response to changes in pO2, hemoglobin ensures that oxygen is efficiently
delivered to tissues where it is needed and released in areas of low pO2. This
behavior of hemoglobin follows Le Chatelier's principle, as it adjusts its
equilibrium position in response to changes in the concentration of oxygen to
maintain a balance in oxygen delivery throughout the body.
Q.8.What is Active mass?
Ans. Active mass, also known as the effective concentration,
is the concentration of the species in a reaction that is directly involved in
the chemical reaction. It is the concentration of the species that appears in
the rate equation or the equilibrium constant expression.
In a chemical reaction, not
all species present may be actively participating in the reaction. Only the
reactants that collide with each other and successfully undergo a chemical
change contribute to the rate of the reaction or affect the equilibrium
position. Therefore, it is essential to consider the active or effective
concentrations of the reacting species when analyzing reaction kinetics or equilibrium.
Active mass is usually represented by square brackets ([ ]) in chemical
equations to distinguish it from other concentrations that may not be directly
involved in the reaction.
Q.9.What are electrolytes and
non-electrolytes?
Ans. Electrolytes are substances that, when dissolved in water
or other solvents, dissociate into ions and conduct electricity. They are
capable of conducting electric current due to the presence of free ions in the
solution. Examples of electrolytes include salts, acids, and bases.
Non-electrolytes, on the
other hand, are substances that do not dissociate into ions when dissolved in
water or other solvents. They do not conduct electricity because they do not
produce free ions in the solution. Examples of non-electrolytes include most
organic compounds like sugars, alcohols, and organic acids.
Q.10.Discuss different types of
electrolytes?
Ans. Electrolytes can be categorized into three main types
based on the nature of their ions when dissolved in water:
Strong
Electrolytes: Strong electrolytes
are substances that completely dissociate into ions when dissolved in water.
They conduct electricity efficiently due to the high concentration of ions.
Examples include strong acids (e.g., HCl, H2SO4, HNO3) and strong bases (e.g.,
NaOH, KOH).
�
�
�
(
�
�
)
→
�
+
(
�
�
)
+
�
�
−
(
�
�
)
HCl (aq) →H
+
(Aq)+Cl
−
(Aq)
�
�
�
�
(
�
�
)
→
�
�
+
(
�
�
)
+
�
�
−
(
�
�
)
NaOH(aq)→Na
+
(Aq)+OH
−
(Aq)
Weak
Electrolytes: Weak electrolytes are
substances that only partially dissociate into ions when dissolved in water.
They conduct electricity to a lesser extent than strong electrolytes due to a
lower concentration of ions. Examples include weak acids (e.g., acetic acid,
CH3COOH) and weak bases (e.g., NH4OH).
�
�
3
�
�
�
�
(
�
�
)
⇌
�
+
(
�
�
)
+
�
�
3
�
�
�
−
(
�
�
)
CH
3
COOH (aq) ⇌H
+
(Aq)+CH
3
COO
−
(Aq)
�
�
4
�
�
(
�
�
)
⇌
�
�
4
+
(
�
�
)
+
�
�
−
(
�
�
)
NH
4
OH (aq) ⇌NH
4
+
(Aq)+OH
−
(Aq)
Nonelectrolytes: Nonelectrolytes are substances that do not dissociate
into ions when dissolved in water. They do not conduct electricity because
there are no free ions present. Examples include most organic compounds such as
sugars (e.g., glucose, sucrose), alcohols (e.g., ethanol), and urea.
�
6
�
12
�
6
(
�
�
)
C
6
H
12
O
6
(Aq)
�
2
�
5
�
�
(
�
�
)
C
2
H
5
OH(aq)
Understanding the different
types of electrolytes is important in various applications, such as in
determining the electrical conductivity of solutions and understanding the
behavior of ionic substances in chemical reactions.
Q.11.Explain why an aqueous solution of
a salt of weak base and strong acid is acidic in nature?
Ans. An aqueous solution of a salt of a weak base and a strong
acid is acidic in nature due to the hydrolysis of the salt.
When a salt of a weak base
and a strong acid dissolves in water, it dissociates into its constituent ions.
The cation comes from the weak base, and the anion comes from the strong acid.
Let's take an example
of ammonium chloride (NH4Cl) as the salt:
�
�
4
�
�
→
�
�
4
+
+
�
�
−
NH
4
Cl→NH
4
+
+Cl
−
The chloride ion (Cl^-) is
derived from the strong acid (HCl), and it does not undergo any significant
hydrolysis. However, the ammonium ion (NH4^+) is derived from the weak base
ammonia (NH3), which can react with water:
�
�
4
+
+
�
2
�
⇌
�
�
3
+
�
3
�
+
NH
4
+
+H
2
O⇌NH
3
+H
3
O
+
In this reaction, water acts
as an acid, and it donates a proton (H+) to the ammonium ion, forming ammonia
(NH3) and a hydronium ion (H3O^+).
Since the solution contains
an excess of hydronium ions (H3O^+), it is acidic in nature. Thus, the overall
acidity of the solution is due to the hydrolysis of the salt and the presence
of hydronium ions from the weak base.
Q.12.What is the law of chemical
equilibrium?
Ans. The law of chemical equilibrium, also known as the law of
mass action, states that the rate of a chemical reaction at equilibrium is
proportional to the product of the concentrations (or partial pressures) of the
reactants, each raised to the power of their respective stoichiometric
coefficients in the balanced chemical equation. It is represented by the
equilibrium constant (Kc for concentrations or Kp for partial pressures) and
expresses the relationship between the concentrations (or partial pressures) of
products and reactants at equilibrium.
Q.13.What do you understand by chemical
equilibrium? Give its four important characteristics?
Ans. Chemical equilibrium is a state in a reversible chemical
reaction where the forward and reverse reactions occur at the same rate,
resulting in a constant concentration of products and reactants over time.
Four important
characteristics of chemical equilibrium are:
Constant
Concentrations: At
equilibrium, the concentrations of products and reactants remain constant.
Although the forward and reverse reactions are still occurring, the rates are
equal, leading to a balance between the amounts of reactants and products.
Dynamic
Process: Chemical equilibrium
is a dynamic process, meaning that the reactions have not stopped; rather, they
are occurring at the same rate in both directions. The concentrations of
reactants and products are stable, but the molecules continue to collide and
interconvert.
Reversible
Reaction: Equilibrium is
achieved in reversible reactions, where reactants can form products and
products can also react to form reactants. A double arrow (
⇌
⇌)
is used to indicate a reversible reaction.
Independent
of Initial Conditions: The
position of equilibrium, determined by the equilibrium constant (
�
�
K
c
or
�
�
K
p
), is independent of the initial
concentrations or pressures of the reactants and products. As long as the
temperature remains constant, any starting concentrations will eventually reach
the same equilibrium concentrations. However, the time taken to reach
equilibrium may vary depending on the initial conditions.
Understanding the characteristics
of chemical equilibrium is essential in various applications, such as
controlling reaction yields, designing chemical processes, and explaining the
behavior of chemical systems in nature and industry.