Chapter 12 Thermodynamics
CHAPTER NO.12 THERMODYNAMICS
12.1 INTRODUCTION
In previous chapter we have studied thenmnal
properties of Inatter. In this chapter we shall study laws that govern.
thermal energy. We shall study the processes where
work is canverted into heat and vice versa. In winter, when we rub our palms
together, we fee] warmer; here work done in rubbing produces the ‘heat’.
Conversely, in a steam engine, the ‘heat’of the steam is used to do useful work
in moving the pistons,
which in turn rotate the wheels of the train.
In physics, we need to deine the notions of
heat,temperature, work, etc. more carefully. Historically, it took a
lang time to arrive at the proper concept of ‘heat’.
Before the
modern picture, heat was regarded as a fine
invisible fluid filling in the pores ofa substance. On contact between a hot
body and a cold bedy, the fluid (called caloric) flowed from the colder to the
hotter body ! This is similar to what happens when a horizontal pipe connects
two tanks containing water
up to different heights. The flow continues until
the kvels of water in the two tanks are the same. Likewise, in the
‘caloric’picture of heat, heat flows until the ‘caloric levels’ (i-e., the
temperatures) equalise.
In time, the picture of heat as a fluid was
discarded in favour of the modern concept of heat as a form of energy. An
important experiment in this connection was due to
Benjamin Thomson {also known as Count Rumford) in 1798. He obseryed that boring
of a brass cannon generated a lot of heat, indeed enough to boil water. More
significantly, the amount of heat produced depended on the work done {by the
horses employed for turning the drill) but not on the sharpness of the drill.
In the caloric picture, 4 sharper drill would scoop out more heat fluid from
the pores; but this was not observed. A most natural explanation of the
observations was that heat was a form of enengy and the experiment demonstrated
conversion of energy from one form to another—irom work to heat.
Thermodynamics is the branch of physics that deals
with the concepts of heat and temperature and the inter-conversion of heat and
other forms of energy. Thermodynamics is a macroscopic
science. [t deals with bulk systems and does not go
into the molecular constitution of matter. In fact, its concepts and laws were
formulated in the
nineteenth century before the molecular picture of
matter was finnly established. Thermodynamic description involves relatively
few macroscopic
variables of the system, which are suggested by
common sense and can be usually measured directly. A microscopic description of
a gaa, for example, would involve specifying the co-ordinates
and velocities of the huge number of molecules
constituting the gas. The description in kinetic theory of gases is not so detailed
but it does hivolve
molecular distribution of velocities.
Thermodynamic description ofa gas, on the other
hand, avoids the molecular description altogether.Instead, the state of a gas
in thermodynamics is
specified by macroscopic variables such as pressure,
vohume, temperature, mass and composition that are felt by our sense
perceptions and are measurable*.
The distinction between mechanics and
thermodynamics is worth bearing in mind. In
mechantks, our interest is in the motion of particles or bodies under the
action of forces and torques.Thermodynamics is not concerned with the motion of
the system as a whole. It is concerned with the internal macroscopic state of
the body.When a bullet is fired from a gum, what changes
is the mechanical state of the bullet (its kinetic
energy, in particular}, not its temperature. When the bullet pierces a wood and
stops, the kinetic energy of the bullet gets converted into heat,
changing the termperature of the bullet and the
surrounding Iayers of wood. Temperature is Telated to the energy of the
internal (disordered)motion of the bullet, not to the motion of the bullet
as a whole.
12.2 THERMAL EQUILIBRIUM
Equilibrium in mechanics means that the net extemal
force and torque on a system are zero.The term ‘equilibriuun’ in
thenmodynaniiks appears ina different context : we say the state ofa system
is an equilibrium state if the macroscopic variables
that characterise the system do not change in time. For example, a gas tnside a
closed
rigid container, completely insulated from its
surroundings, with fixed values of pressure,volume, temperature, mass and
composttion that do not change with time, is in a state of thermodynamic
equilibrium.
In general, whether or not a system is ina state of
equilibrium depends on the surroundings and the nature of the wall that
separates the system from the surroundings. Consider two gases Aand
B occupying two different containers. We know
experimentally that pressure and volume of a given maas of gas can be chosen to
be its two independent variables. Let the pressure and
volume of the gases be {P, Vv, and (P,,
V,)respectively. Suppose first that the two systems are put in proximity but
are separated by an adiabatic wall — an insulating wall (can be movable} that
does not allow flow of energy (heat)
from one to another. The systems are insilated from
the rest of the surroundings also by similar adiabatic walls. The situation {s
shown
schematically in Fig. 12.1 {a). In this case, it is
found that any possible pair of vahies (P,, V,) will be in equilfbriim with any
poasible pafr of valies (P,, V,,}. Next, suppose that the adiabatic wall is
replaced by 4 diathermic wall —a conducting wall
that allows enerpy flow (heat) fram one to
another.It is then found that the macroscopic variables of the systems A and
Bchange spontaneously until
both the syatems attatn equittbrium states. After
that there is no change in their states. The situation is shown in Fig.
12.1(b). The pressure and vohume variables of the two gases change to
(P,‘, V,) and (P,’, V,’) such that the new states of
A and Bare in equilfprtum with each other**.There is no more energy flow from
one to another.
We then say that the system A is in thermal
equilibrium with the system B.
What characterises the sttuation of thermal
equilibrium between two systems ? You can guess the answer from your
experience. In thermal equilibrium, the temperatures of the two systems
are equal. We shall see how does one arrive at the
concept of temperature in thermodynamics?The Zeroth law of thermodynamics
provides the clue.
12.3 ZEROTH LAW OF THERMODYNAMICS
Imagine two systems A and B, separated by an
adiabatic wall, wile each is in contact with a third system C, via a conducting
wall [Fig. 12.2{a)]. The
states of the systems (Le., their macroscopic variables)
will change wntil both A and Bcome to thermal equilibrium with C. After this is
achieved,suppose that the adiabatic wall between Aand B
is replaced by a conducting wall and Cis insulated
from Aand Bby an adiabatic wall [Fig.12.2(b)]. It is found that the states of A
and B change no further Le. they are found toe be in thermal
equilibrium with each other. This observation forms
the basis of the Zeroth Law of Thermodynamics, which states that ‘two systems
in thermal equilibrium with a third system separately are in thermal
equilibrium
with each other’. RH. Fowler formulated this law in
1931 long after the first and second Laws of thermodynamics were stated and so
numbered.
The Zeroth Law clearly suggests that when two
systems A and B, are in thermal equilibrium,there must be a physical quantity
that has the same value for both. This thermodynamic variable whose value is
equal for two systems in
thermal equilibrium is called temperature (T).Thus,
if A and B are separately in equilibrium with C, T, =Te and Tz = Te. This
implies that
T, = Ty 1.e. the systems A and B are also in thermal
equilibrium.
‘We have arrived at the concept of temperature
formally via the Zeroth Law. The next question is: how to assign numerical
values to temperatures of different bodies? In other words,
how do we construct a scale of temperature
?Thermometry deals with this basic question to which we turn in the next
section.
12.4 HEAT, INTERNAL ENERGY AND WORK
The Zeroth Law of Thermodynamics led us to the
concept of temperature that agrees with our commonsense notion. Temperature is
a marker of the ‘hotness’ of a body. It determines the direction of flow of
heat when two bodies are
placed in thermal contact. Heat flows from the body
at a higher temperature to the one at lower temperature. The flow stops when
the temperatures equalise; the two bodies are then in thermal equilibrium. We
saw in some detail
how to construct temperature scales to assign
temperatures to different bodies. We now describe the concepts of heat and
other relevant quantities ke internal energy and work.
The concept of internal energy of a syatem is not
difficult to understand, We know that every bulk system consists of a large
number of molecules. Internal energy is amply the sum of the kinetic energies
and potential enerpies of
these molecules. We remarked earler that in
thermodynamics, the kinetic energy of the system, as a whole, is not relevant.
Internal energy is thus, the sum of molecular kinetic and potential energies in
the frame of reference Telative to which the centre of mass of the system
is at rest. Thus, it includes only the
(disordered)energy associated with the random motion of molecules of the
system. We denote the internal
energy of a system by U.
Though we have invoked the molecular
picture to understand the meaning of internal
energy, as far as thermodynamics is concerned,Uis stmply a macroscopic variable
of the system.
The important thing about internal energy is that it
depends only on the state of the system,not on how that state was achieved.
Internal energy U of a system is an example of a thermodynamic ‘state variable’
— its value depends only on the given state of the system.not on history Le.
not on the ‘path’ taken to arrive at that state. Thus, the internal energy ofa
given
mass of gas depends on its state described by
specific values of pressure, volume and temperature. It does not depend on how
this state of the gas came about Pressure, volume,
temperature, and internal energy are
thermodynamic state variables of the system (gas)
(see section 12.7). if we neglect the small intermolecular forces in a gas, the
internal energy of a gas is just the sum of kinetic energies
associated with various random motions of its
molecules. We will see tn the next chapter that in a gas this motion is not
only translational (i.e. motion from one point to another in the
volume of the container); it alsc includes
rotational and vibrational motion of the molecules (Fig. 12.3).
What are the ways of changing internal
energy of a system ? Consider again, for
simplicity, the system to be a certain mass of gas
contained in a cylinder with a movable piston as shown in Fig. 12.4. Experience
shows there are two ways of changing the state of the gas (and hence its
internal energy). One way is
to put the cylinder in contact with a body at a
higher temperature than that of the gas. The temperature difference will cause
a flow of energy (heat) from the hotter body to the gas,thus fucreasing the
internal energy of the gas.The other way is to push the piston down t.e. to do
work on the system, which again results in increasing the internal energy of
the gas. Of
course, both these things could happen in the
reverse direction. With surroundings at a lower temperature, heat would flow
from the gas to
the surroundings. Likewise, the gas coukl push the
piston up and do work on the surroundings.In short, heat and work are two
different modes of altering the state ofa thermodynamic system
and changing its internal energy.
The notion of heat should be carefully
distinguished from the notion of internal
energy.Heat is certainly energy, but it is the energy in
transit. This is not just a play of words. The
distinction is of basic significance. The state of a thermodynamic system is
characterised by its internal energy, not heat. A statement like ‘a gas in a
given state has a certain amount of heat’ is as meaningless as the statement
that
‘a gas in a given state has a certain amount of
work’. In contrast, ‘a gas in a given state has a certain amount of internal
energy’ is a perfectly meaningful statement. Stmilarly, the
Statements ‘a certain amount of heat is
supplied to the system’ or ‘a certain amount of work
was done by the system’ are perfectly meaningful.
To summarise, heat and work in
thermodynamics are not state variables. They are
modes of energy transfer to a system resiuting in change in ite internal
energy,which, as already mentioned, is a state variable.In ordinary language,
we often confuse heat with internal energy. The distinction between them is
sometimes ignored in elementary physics books. For proper understanding of
thermodynamics, however, the distinction s crucial.
12.6 FIRST LAW OF THERMODYNAMICS
We have seen that the internal energy U of a system
can change through two modes of energy transfer : heat and work. Let
AQ = Heat supplied to the system by the
surroundings AW = Work done by the system on the
surroundings AU = Change in internal energy of the system
The general principle of conservation of
energy then implies that AQ = AU+AW (12.1) i.e. the
energy (AG) supplied to the syatem goes in partly to increase the internal
energy of the system (AU) and the rest in work on the environment (AW).
Equation (12.1) is known as
the First Law of Thermodynamics. It is simply the
general law of conservation of energy applied to any system in which the energy
transfer from or to the surroundings is taken into account.
Let us put Eq. (12.1) in the alternative form AQ -
AW =AU (12.2)
Now, the system may go from an initial state to the
final state in a number of ways. For example, to change the state of a gas from
(P,. V,) to (P,, V). we can firat change the volume of the gas from V, to V,,
keeping its pressure constant 1.c. we can first go the state
(P,. V,) and then change the preasure of the gas
from P, to P,, keeping volume constant, to take the gas to (P,. V,).
Alternatively, we can first keep the volume constant and then keep
the pressure constant. Since U is a atate variable,
AU depends only on the initial and final states and not on the path taken by
the gas to go from one to the other. However, AQ and AW will, in general,
depend on the path taken to go from the initia] to final states. From
the Firat Law of Thermodynamics, Eq. (12.2).it is
clear that the combination AG - AW, is however, path independent. This shows
that tifa system is taken through a process in which AU = 0 (for example,
isothermal expansion of
an ideal gas, see section 12.8),
AQ =AW 1e., heat supplied to the system is used up
entirely by the system in doing work on the environment.
If the system is a gas in a cylinder with a movable
piston, the gas in moving the piston doea work. Since force is preasure times
area,and area times diaplacement is volume, work done by the system against a
constant pressure
Pis
AW =PAV
where AV is the change in volume of the gas.Thus,
for this case, Eq. (12.1) gives AQ = AU+ PAV (12.3)
As an application of Eq. (12.3), consider the change
in internal energy for 1 ¢ of water when we go from its liquid to vapour phase.
The measured latent heat of water is 2256 J/g. i.e.,
for 1 g of water AQ = 2256 J. At atmospheric
pressure, 1 g of water has a volume | cm? in liquid phase and 1671 cm? in
vapour phase.Therefore,AW=P(V,-V,) = 1.013 x10° x(1670)x10° =169.2J Equation
(12.3) then gives AU= 2256 - 169.2 = 2086.87 We see that most of the heat goes
to increase the internal energy of water in transition from the liquid to the
vapour phase.
12.6 SPECIFIC HEAT CAPACITY
Suppose an amount of heat AQ supplied to a substance
changes its temperature from T to T+ AT. We define heat capacity of a substance
(2ee Chapter 11) to be
249 Saar (12.4)
We expect AQ and, therefore, heat capacity S to be
proportional to the mass of the substance.Further, it could also depend on the
temperature, 1.¢., a different amount of heat may be needed for a untt rise in
temperature at
different temperatures. To define a constant
characteristic of the substance and independent of its amount, we divide S by
the mass of the substance m in kg:
— 8 _ f1\ag
s= a= (— Ke (12.5)
8 is known as the specific heat capacity of the
substance. It depends on the nature of the substance and its temperature. The
unit of specific heat capacity is Jkg?} K1.If the amount of substance is
specified in terms of moles p (instead of mass min kg), we can define heat
capacity per mole of the
substance by S 1aAQ9 Cc WAT (12.6)
C is known as molar specific heat capacity of the
substance. Like s, C is independent of the amount of substance. C depends on
the nature of the substance, its temperature and the
conditions under which heat is supplied. The unit of
C is J mol" K". As we shall see later (in connection with spectfic
heat capacity of gases),additional conditions may be needed to define
C or s. The idea in defining C is that simple
predictions can be made in regard to molar specific heat capacities.
Table 12.1 lists measured specific and molar heat
capacities of solids at atmospheric pressure and ordinary room temperature.We
will see in Chapter 13 that predictions of specific heats of gases generally
agree with experiment. We can use the same law of equipartition of energy that
we use there to
predict molar specific heat capacities of
solids.Consider a solid of N atoms, each vibrating about its mean position. An
oscillator in one dimension has average energy of 2 x % kT = k,T. In three
dimensions, the average energy
is 3 k,T. Fora mole ofa solid, the total energy is U =3k,T xN, =3RT
Now, at constant pressure, AQ = AU + PAV=
AU, since for a solid AV 1s negligible. Therefore,
c
AQ _AU gn 12 AT AT 02.7)
Table 12.1 Specific and molar heat capacities of
some solids at room
temperature and atmospheric
pressure
As Table 12.1 shows, the experimentally
Measured values which generally agrees with predicted value 3R at ordinary temperatures.
(Carbon is an exception.) The
agreement is imown to break down at low temperatures.Specific heat capactty of
water The old unit of heat was calorie. One calorie was earlier defined to be
the amount of heat required to raise the temperature of 1g of water
by 1°C. With more precise measurements, tt was found
that the specific heat of water varies slightly with temperature. Figure 12.5
showa this variation in the temperature range 0 to 100°C.
For a precise definition of calorie, it
was,therefore, necessary to specify the unit temperature interval. One calorie
is defined to be the amount of heat required to raise the temperature of 1g of
water from 14.5 °C to 15.5 °C. Since heat is just a form of energy.it 1a
preferable to use the unit joule, J.
In SI units, the specific heat capacity of water is
4186 J kg! K' i.e. 4.186 J g! K". The so called mechanical equtvalent of
heat defined as the amount of work needed to produce 1 cal of heat is in fact
just a conversion factor
between two different units of energy : calorie to
joule. Since in SI units, we use the unit joule for heat, work or any other
form of energy, the term mechanical equivalent is now
superfluous and need not be used.
As already remarked, the specific heat
capacity depends on the procesa or the
conditions under which heat capacity transfer takes
place. For gases, for example, we can define two specific heats : specific heat
capacity at constant volume and specific heat capacity at constant pressure.
For an
ideal gas, we have a simple relation.
C,- C,=R (12.8)where C, and C, are molar specific
heat capacities of an ideal gas at constant pressure and vohame respectively
and R is the universal
gas constant. To prove the relation, we begin with
Eq. (12.3) for 1 mole of the gas :AQ =AU+ PAV If AQ is absorbed at constant
volume, AV =0 «-(22) ~(a4) (28)
& ( aT}, Var}, Var (12.9)
where the subscript v is dropped in the last step,
since U of an ideal gas depends only on temperature. (The subscript denotes the
quantity kept fixed.) If, on the other hand, AQ
is absorbed at conatant pressure,
Ty (AQ) — (AU AV )
c= (Rl (Gel rar] azo
The subscript p can be dropped from the
first term since U of an ideal gas depends only on
T. Now, for a mole of an ideal gas PV=RT which gives 1 (AV
° (7), =k (12.11) Equations (12.9) to (12.11) give
the desired Telation, Eq. (12.8).
12.7 THERMODYNAMIC STATE VARIABLES
AND EQUATION OF STATE
Every equilibrium state of a thermodynamic system is
completely described by specific values of some macroscopic variables, also
called state variables. For example, an equilibrium state of a gas is
completely specified by the values of pressure, volume,
temperature, and mass (and composition if there is a
mixture of gases). A thermodynamic syatem is not always in equilibrium. For
example,a gas allowed to expand freely against vacuum
is not an equilibrium state [Fig. 12.6{a}]. During
the rapid expansion, preasure of the gaa may not be untform throughout.
Similarly, a mixture
of gases undergoing an explosive chemical reaction
(e.g. a mixture of petrol vapour and air when ignited by a spark) is not an
equilibrium state; again its temperature and pressure are not wniform [Fig.
12.6(b)].Eventually, the gaa attains a uniform temperature and pressure and
comes to thermal and mechanical equilibrium with its
surroundings.
In short, thermodynamic state variables
describe equilibrium states of systems. The various
state variables are not necessarily independent. The connection between the
state variables is called the equation of state. For example, for an ideal gas,
the equation of state
is the ideal gas relation
PV=pRT
For a fixed amount of the gas i.e. given p, there
are thus, only two independent variables, say P and V or Tand V. The
pressure-volume curve for a fixed temperature is called an isotherm.
Real gases may have more complicated
equations of state.
The thermodynamic state variables are of two kinds:
extensive and intensive. Extensive variables indicate the ‘size’ of the
system.Intensive variables such as pressure and temperature do not. To decide
which variable is extensive and which intensive, think of a relevant system in
equilibrium, and imagine that
it 1s divided into two equal parts. The variables
that remain unchanged for each part are intensive. The variables whose values
get halved in each part are extensive. It is easily seen, for
example, that internal energy U, volume V, total
mass M are extensive variables. Pressure P,temperature T, and density p are
intensive variables. It is a good practice to check the consistency of
thermodynamic equations using this clasatfication of variables. For example, in
the equation AQ=AU+ PAV quantities on both sides are extensive®. (Theproduct of
an intensive variable like P and an extensive quantity AV is extensive.)
12.8 THERMODYNAMIC PROCESSES
12.8.1 Quasi-static process
Consider a gas in thermal and mechanical
equilibrium with its surroundings. The pressure of
the gas in that case equals the external pressure and its temperature is the
same as that of its surroundings. Suppose that the external pressure is
suddenly reduced (say by lifting the weight on the movable piston in the
container). The piston will accelerate outward.During the process, the gas
passes through states that are not equilfbriium states. The non-
equilibrium states do not have well-defined pressure
and temperature. In the same way, if a finite temperature difference exists
between the gas and its surroundings, there will be a rapid exchange of heat
during which the gas
will pass through non-equilibrium states. In due
course, the gas will settle to an equilibrium state with well-defined temperature
and
presaure equal to those of the surroundings. The
free expansion ofa gas in vacuum and a mixture of gasea undergoing an explosive
chemical
reaction, mentioned in section 12.7 are also
examples where the system goes through non-equilibrium states.
Non-equilibrium states of a system are difficult to
deal with. It is, therefore, convenient to imagine an idealised process in
which at every
stage the system is an equilibrium atate. Such a
process is, in principle, infinitely slow-hence the name quasi-static (meaning
nearly static). The
system changes its variables (P, T, V) so slowly
that {t remains in thermal and mechanical equilibrium with fis aurroundings
throughout.In a quasi-static process, at every stage, the
difference in the pressure of the system and the
external pressure is infinitesimally small. The same is true of the temperature
difference
between the syatem and its surroundings. To take a
gas from the state (P, T) to another state (P’. T’)} via a quasi-static
process, we change the external pressure by a very small amount,
allow the system to equalise its pressure with that
of the surroundings and continue the process infinitely slowly until the system
achieves the pressure P’. Similarly, to change
the temperature, we introduce an infinitesimal
temperature difference between the system and the surrounding reservoirs and by
choosing reservoirs of progressively different temperatures
T to T’, the system achieves the temperature T’.A
quasi-static process is obviously a hypothetical construct. In practice,
processes that are sufficiently slow and do not involve
accelerated motion of the piston, large
temperature gradient, etc. are reasonably
approximation to an ideal quasi-static proceas.We shall from now on deal with
quasi-static processes only, except when stated otherwise.
A process in which the temperature of the system is
kept fixed throughout is called an isothermal process. The expansion ofa gas in
a metallic cylinder placed in a large reservoir of
fixed temperature is an example of an isothermal
process. {Heat transferred from the reservoir to the system does not materially
affect the temperature of the reservoir, becanse of its very
large heat capacity.) In isobaric processes the
pressure is constant while in isochoric processes the volume is constant.
Finally, if the system is insulated from the surroundings
and no heat flows between the system and the
surroundings, the process is adiabatic. The definitions of these special
processes are summarised in Table. 12.2 Table 13.2 Some special thermodynamic
processes
We now consider these processes in some detail
:Isothermal process For an isothermal process (T fixed), the ideal gas equation
gives PV = constant
Le., pressure ofa given mass of gas varies inversely
as its volume. This is nothing but Boyle’s Law.
Suppose an ideal gas goes isothermally (at
temperature T) from its initial state (P,. V,) to the final state (P,. V,). At
any intermediate stage with pressure P and volume change from V to
V+ AV (AV small)AW = PAV Taking (AV — 0) and summing
the quantity AW over the entire process,oy We] Pdv V,, Vv.
2dv Vv, wee > = BERT ns (12.12)
where in the second step we have made use of the
ideal gas equation PV =p) RT and taken the constants out of the integral. For
an ideal gas,internal energy depends only on temperature.
Thus, there is no change in the internal energy of
an ideal gas in an isothermal procesa. The First Law of Thermodynamics then
implies that heat supplied to the gas equals the work done by the gas : Q = W.
Note from Eq. (12.12) that
for V,> V,, W> 0; and for V,< V,, W<0.
That is, in an isothermal expansion, the gas absorbs heat and doea work while
in an isothermal compression, work is done on the gas by the environment and
heat ts released.
Adiabatic process
In an adiabatic process, the system is insulated
from the surroundings and heat absorbed or Teleased is zero. From Eq. (12.1),
we see that work done by the gas results in decrease in its internal energy
(and hence its temperature for
an ideal gas). We quote without proof (the result
that you will learn in higher courses) that for an adiabatic proceas of an
ideal gas.PV’ = const (12,13)where y is the ratio of specific heats (ordinary
or molar) at constant pressure and at constant volume.
_P
7 Cy
Thus if an ideal gas undergoes a change in its state
adiabatically from (P,, V,) to (P,, V.) :P vy =P, vy (12.14)
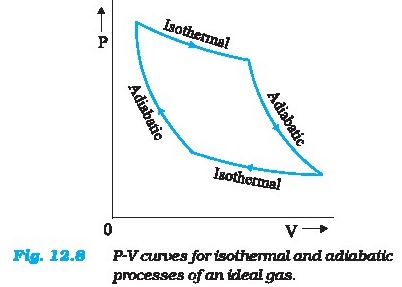
Figure 12.8 shows the P-V curvea of an ideal gas for
two adiabatic processes connecting two isotherms.
We can calculate, aa before, the work done in an
adiabatic change of an ideal gas from the state (P,, V,, T,) to the state (P,,
V,, T,).OY We | PdV Y
V5 dv yor Vv;=constant x } —=constant x — Vv, Vi 1-¥
Vv,constant 1 1 From Eq. (12.34), the constant ts PV," or PV,"
w - J! PVy' JPl-y[ vs v7 _ BRC, -
7.)=[oylevs-PMJ-—~a = a216)
As expected, if work is done by the gas in an
adiabatic process (W > 0}, from Eq. (12.16),T, <T,. On the other hand, if
work is done on the gas (W < 0), we get T, > T, i.c., the temperature of
the gas rises.
Isochoric process
In an isochoric process, Vis constant. No work is
done on or by the gas. From Eq. (12.1), the heat absorbed by the gas goes
entirely to change its internal energy and its temperature. The
change in temperature for a given amount of heat is
determined by the specific heat of the gas at constant volume.
Isobaric process
In an isobaric process, P is fixed. Work done by the
gas is W=PiV,-V) = HR(T,-T) (12.17)
Since temperature changes, so does internal energy.
The heat absorbed goes partly to increase internal energy and partly to do
work.The change in temperature for a given amount
of heat is determined by the specific heat of the
gas at constant pressure.
Cyclic process
In a cyclic process, the system returns to its
initial state. Since internal energy is a state variable, AU = 0 for a cyclic
process. From Eq. (12.1), the total heat absorbed equals the work done by the
system.
12.0 HEAT ENGINES
Heat engine is a device by which a system is made to
undergo a cyclic process that results in conversion of heat to work.
(1) It consists of a working substance-the system.
For example, a mixture of fuel vapour and air in a gasoline or diesel engine or
steam in a steam engine are the working substances.
(2) The working substance goes through a cycle
consisting of several processes. In some of these processes, it absorbs a total
amount of heat Q, from an external reservoir at some high temperature T,,.
(3) In some other processes of the cycle, the
working substance releases a total amount of heat Q, to an external reservoir
at some lower temperature T,.
(4 The work done (W) by the system in a cycle is
transferred to the environment via some arrangement (e.g. the working substance
may be in a cylinder with a moving piston that transfers mechanical energy to
the wheels of a vehicle via a shaft).
The basic features of a heat engine are
schematically represented in Fig. 12.9.
The cycle is repeated again and again to get useful
work for some purpose. The discipline of thermodynamics has its roots in the
study of heat engines. A basic question relates to the efficiency
of a heat engine. The efficiency (n) of a heat
engine is defined by ow
Or (12.18)where Q, is the heat input i.ec., the heat
absorbed by the system in one complete cycle and W is the work done on the
environment in a cycle. Ina cycle, a certain amount of heat (Q,}
may also be rejected to the environment.
Then,according to the First Law of Thermodynamics,over one complete cycle,
w=9,-9, (12.19)
i.e.,
=|-22
7=1 Q1 (12.20)
For 9, = 0, 1 = 1, Le., the engine will have 100%
efficiency in converting heat into work.Note that the Firat Law of
Thermodynamics tLe.,the energy conservation law does not rule out
such an engine. But experience shows that such an
ideal engine with 7 = 1 is never possible,even if we can eliminate various
kinda of loases associated with actual heat engines. It turns out that there is
a fundamental limit on the
efficiency of a heat engine set by an independent
principle of nature, called the Second Law of Thermodynamics (section 12.11).
The mechanism of conversion of heat into
work varies for different heat engines.
Basically,there are two ways : the system (say a gas ora mixture of gases) ia
heated by an external furnace, as in a steam engine; or ft is heated
internally by an exothermic chemical reaction as in
an internal combustion engine. The various steps involved in a cycle also
differ from one engine to another.
12.10 REFRIGERATORS AND HEAT PUMPS
A refrigerator is the reverse of a heat engine.Here
the working substance extracts heat Q,from the cold reservoir at temperature
T,, some external work W is done on it and heat 9, is released to the hot
reservoir at temperature T,
(Fig. 12.10).
Pioneers of Thermodynamics Lond Kelvin (William
Thomson) (1824-1907), born in Belfast, Ireland, is . among the forcmost British
scientists of the nineteenth century. Thomson a< j played a key role in the
development of the law of conservation of energy ans Suggested by the work of
James Joule {1518-1889}, Julius Mayer (1814
f Rd 1878) and Hermann Helmholtz (1821-1894), He collaborated with Joule
7 A rr on the so-called Joule-Thomaon effect : cooling of a gas when it expands
: a ; inte vacuum. He introduced the notion of the absolute zero of
temperature—_ VY and propoeed the abeolute temperuture acale, now called the
Kelvin ecale " * tn his honour. From the work of Sadi Carnot (1796-1832),
Thomson arrived at a form of the Second Law of Thermodynamics. Thomson was a
versatile physiciet, with notable contributions ta clectromagnetic theory and
hydrodynamics.
0 Radolf Claustus (1822-1888), born in Poland, is
generally regarded as
the discoverer of the Second Law of Thermodynamics.
Based an the work
of Camot and Thomson, Clauaiue arrived at the
important notion of entropy
that led bim to a fundamental version of the Second
Law of Thermodynamics that states that the entropy of an isolated system can
never decrease. Clausins also worked on the kinetic theory of gases and
obtained the firat reliable estimates of molecular size, speed, mean free path,
ete.
A heat pump is the same as a refrigerator.What term we use depends on the
purpose of the device. If the purpose is to cool a portion of
apace, like the inside of a chamber, and higher
temperature reservoir is surrounding, we call the device a refrigerator; if the
idea is to pump
heat into a portion of space (the room in a building
when the outside environment is cold),the device is called a heat pump.
In a refrigerator the working substance
(usually, {n gaseons form) goes through the
following steps : (a) sudden expansion of the gas from high to low pressure
which cools it and
converts it into a vapour-liquid mixture,
(b)absorption by the cold fluid of heat from the region to be cooled converting
it into vapour, (c)heating up of the vapour due to external work
done on the system, and (d) release of heat by the
vapour to the surroundings, bringing it to the initial state and completing the
cycle.
The coefficient of performance (a) of a
refrigerator is given by 92 a= (12.21)
where Q, is the heat extracted from the cold
reservoir and W is the work done on the system-the refrigerant. (a for heat
pump is defined as 9, /W) Note that while 7 by definition can never exceed 1, a
can be greater than 1.By energy conservation, the heat released to the
hot reservoir is 9,=W+Q,fe. & “90 (12.22)In a
heat engine, heat cannot be fully converted to work; likewise a refrigerator
cannot work without some external work done on the syatem, i.e., the
coefficient of performance in Eq.
(12.21) cannot be infinite.
12.11 SECOND LAW OF THERMODYNAMICS
The First Law of Thermodynamics is the principle of
conservation of energy. Common experience shows that there are many conceivable
processes that are perfectly allowed by the First
Law and yet are never observed. For example,nohody
has ever seen a book lying on a table jumping to a height by ftself. But such a
thing would be possible if the principle of conservation of energy were the
only restriction. The table could cool spontaneously, converting some of its
internal energy into an equal amount of
mechanical energy of the book, which would then hop
to a height with potential energy equal to the mechanical energy it acquired.
But this never happens. Clearly, same additional basic
principle of nature forbids the above, even though
it satisfies the energy conservation principle. This principle, which disaflows
many phenomena consistent with the First Law of
Thermodynamics is known as the Second Law of
Thermodynamics.
The Second Law of Thermodynamics gives a
fundamental limitation to the efficiency of a heat
engine and the co-efficient of performance of a refrigerator. In simple terms,
it says that efficiency of a heat engine can never be unity.According to Eq.
(12.20). this implies that heat
released to the cold reservoir can never be made
zero. For a refrigerator, the Second Law says that the co-efficient of
performance can never be infintte. According to Eq. (12.21), this implics
that external work (W) can never be zero. The
following two statements, one due to Kelvin and Planck denying the possibility
of a perfect heat
engine, and another due to Clausius denying the
possibility of a perfect refrigerator or heat pump, are a concise summary of
these observations.
Second Law of Thermodynamics Kelvin-Planck
statement.No process is possible whose sole result is the absorption of heat
from a reservoir and the
complete conversion of the heat into work.Clausius
statement No process is possible whoae sole result is the
transfer of heat from a colder object to a hotter
object.It can be proved that the two statements above are completely
equivalent.
123.12 REVERSIBLE AND IRREVERSIBLE PROCESSES
Imagine some process in which a thermodynamic system
goes from an initial state 1 to a final state jf: During the process the system
absorbs
heat 9 from the surroundings and performs work W on it.
Can we reverse this process and bring both the system and surroundings to their
initial states with no other effect anywhere ?
Experience suggests that for most processes in
nature this is not possible. The spontaneous processes of nature are irreversible.
Several
exampks can be cited. The base of a vesse] on an
oven is hotter than its other parts. When the vessel is removed, heat is
transferred from the base to the other parts, bringing the vessel
to a uniform temperature (which in due course cools
to the temperature of the surroundings).The process cannot be reversed; a part
of the vessel will not get cooler spontaneously and warm up the base. It will
violate the Second Law
of Thermodynamics, if it did. The free expansion of
a gas is irreversible. The combustion reaction of a mixture of petrol and air
ignited by 4 spark
cannot be reversed. Cooking gas leaking from a gas
cylinder in the kitchen diffuses to the entire room. The diffusion proceas will
not spontancously reverse and bring the gas back
to the cylinder. The stirring of a Hquid in thermal
contact with a reservoir will convert the work done into heat, increasing the
internal energy of the reservoir. The process cannot be reversed exactly;
otherwise it would amount to conversion of heat entirely into work, violating
the Second
Law of Thermodynamics. Irreversibility is a rule
rather an exception in nature.
Irreversibility arises mainly from two causes:one,
many processes (like a free expansion, or an explosive chemical reaction) take
the aystem to non-equilibrium states; two, mast processes
involve friction, viscosity and other dissipative
effects (e.g., a moving body coming to a stop and losing its mechanical energy
as heat to the floor
and the body, a rotating blade in a Hquid coming to
a stop due to viscosity and losing its mechanical energy with corresponding
gain in the internal energy of the liquid). Since
disstpative effects are present everywhere and can
be minimised but not fully eliminated, most processes that we deal with are
irreversible.
Athermodynamic process (state !— state f)is
reversible if the process can be turned back such that both the system and the
surroundings return to their original states, with no other
change anywhere else tn the universe. From the
preceding discussion, a reversible process is an idealised notion. A process is
reversible only if
itis quasi-static (system in equilibrium with the
surroundings at every stage) and there are no dissipative effects. For example,
a quasi-static isothermal expansion of an ideal gas in a
cylinder fitted with a frictionless movable piston
is a reveraible process.
Why is reversibility such a basic concept in
thermodynamics 7? As we have seen, one of the concerns of thermodynamics is the
efficiency
with which heat can be converted into work.The
Second Law of Thermodynamics rules cut the possibility of a perfect heat engine
with 100% efficiency, But what is the highest efficiency
possible for a heat engine working between two
reservoirs at temperatures T, and T,? It turns out that a heat engine based on
idealised reversible processes achieves the highest
efficiency possible. All other engines involving
irreversibility in any way (as would be the case for practical engines) have
lower than this Imiting efficiency.
12.13 CARNOT ENGINE
Suppose we have a hot reservotr at temperature T,
and a coki reservoir at temperature T,. What is the maxtnum efficiency possible
for a heat
engine operating between the two reservoirs and what
cycle of processes should be adopted to achieve the maximum efficiency ? Sadi
Carnot,a French engineer, first considered this question
in 1824. Interestingly, Carnot arrived at the
correct answer, even though the basic concepts of heat and thermodynamics had
yet ta he firmly
established.
We expect the ideal engine operating between two
temperatures to be a reversible engine.lrreversibility is associated with
dissipative effects, as remarked in the preceding section,
and lowers efficiency. A process is reversible if it
ia quaai-static and non-disaipative. We have seen that a process is not
quasi-static if it
involves finite temperature difference between the
system and the reservoir. This implies that in a reversible heat engine
operating between two temperatures, heat should be absorbed
(from the het reservoir) isothermally and released
(to the cold reservoir) isothermally. We thus have identified two steps of the
reversible heat engine : isothermal process at temperature
T, absorbing heat 9, from the hot reservoir, and
another isothermal process at temperature T,releasing heat Q, to the cold
reservoir. To complete a cycle, we need to take the system from temperature T,
to T, and then back from
temperature T, to T,. Which processes should we
employ for this purpose that are reversible?
A little reflection shows that we can only adopt
reversible adiabatic processes for these purposes, which involve no heat flow
from any
reservoir. If we employ any other proceas that is
not adiabatic, say an isochoric process, to take the system from one
temperature to ancther, we
shall need a series of reservoirs in the
temperature range T, to T, to ensure that at cach
stage the process is quasi-static. (Remember again that for a process to be
quasi-static and
reversible, there should be no finite temperature
difference between the system and the reservoir.)But we are considering a
reversible engine that
operates between only two temperatures. Thus
adiabatic processes must bring about the temperature change in the system from
T, to T,and T, to T, in this engine.
A reversible heat engine operating between two
temperatures is called a Carnot engine. We have just argued that such an engine
must have the following sequence of steps constituting one
cycle, called the Carnot cycle, shown in Fig. 12.11.
We have taken the working substance of the Carnot engine to be an ideal gus.
(a) Step 1 > 2 Isothermal expansion of the gas
taking its state from (P., V,, T,) to (P,, V,, T).The heat absorbed by the gas
(Q,) from the reservoir at temperature T, is given by Eq, (12.12). This is also
the work done (W, ,.)
by the gas on the environment.1,Ws = 9, =HRT, In
(x4) (12.23)(b) Step2-—3 Adiabatic expanaton of the gas from (P,, V,. T;) to
(P,, Vy. T;)Work done by the gas, using Eq. (12.16), is wy HRD -T.|
Ws a (12.24)(c) Step3— 4 Isothermal compression of
the gas from (P,, V,, T,) to (P,, V,, T,).Heat released (Q,) by the gas to the
reservoir at temperature T, is given by Eq. (12.12). This
is also the work done (W, _, ,) on the gas by the
environment.. ly,Ws.4 = Q = wRTeAn| (12.25). a (@) Step4—1 Adtabatic compression
of the gas from (P, V,, T,) to (P,.V,. T).Work done on the gaa, [using
Eq.(12.16)], is (T, -T;)
Wi. =uR “= =e | (12.26)From Eqs. (12.23) to (12.26)
total work done by the gas in one complete cycle is W=W, 2 + Wo3-Ws 44-Wasi Vp
Ws = RT, In (i) —- HRT, im( 7) (12.27)The efficiency 7 of the Carnot engine is
Me g, 9,. n( Ze _ Ty V, ?
=! -( 2 nas (12.28)nl Now since step 2 —> 3 is an
adiabatic process,, gel vol
T, Vy =T) Vy , Gen 2 ({hLe. V, = ( T, |
(12.29)Similarly, since step 4 — 1 is an adiabatic process T, Vy m =f, v
‘ ylfyel le. = & (12.80)e. Vv; 7, | .
From Eqs. (12.29) and (12.30), ‘Va _V;
Tv (12.31)Using Eq. (12.31) in Eq. (12.29), we get .
r nel “"7, (Camot engine) (12.32)We have already seen that a Carnot engine
is a reveraible engine. Indeed ft is the only reversible engine possible that
works between two reservoirs at different temperatures. Each step of the Carnot
cycle given in Fig. 12.11 can be reversed. This will amount to taking heat
9,from the cold reservoir at T,, doing work W on
the system, and transferring heat Q, to the hot
Teservoir. This will be a reverafble refrigerator.
We next establish the important result
(sometimes called Carnot’s theorem) that
(a) working between two given temperatures T,and T,
of the hot and cold reservoirs respectively,no engine can have efficiency more
than that of
the Carnot engine and {b) the efficiency of the
Carnot engine is independent of the nature of the working substance.
To prove the result (a), imagine a reversible
(Carnot) engine R and an irreversible engine I working between the same source
(hot reservotr)and sink (cold reservoir}. Let us couple the
engines, I and R, in such a way so that I acts like
a heat engine and R acts as a refrigerator.Let I absorb heat Q, from the
source, deliver work W’ and release the heat 9,- W to the sink.We arrange so
that R returns the same heat 9,to the source, taking heat O, from the sink and
reauiring work W = O. = OQ. to be done on tt.
Now suppose ny < 7, 1c. if R were to act
as an engine it would give less work output than
that of lic. W< W’ fora given 9,. With R acting like a refrigerator, this
would mean
9,= 9.-W> QO, -W’. Thus on the whole,
the coupled I-R system extracts heat
(9, -W- ©, - W) = (W’ - W) from the cold
reservoir and delivers the same amount of work in
one cycle, without any change in the source or anywhere else. This is clearty
against the Kelvin-Planck statement of the Second Law of
Themnodynamics. Hence the assertion 7, > ti,is
wrong. No engine can have efficiency greater than that of the Carnot engine. A
similar argument can be constructed to show that a reversible engine with one
particular substance
cannot be more efficient than the one using another
substance. The maximum efficiency of a Carnot engine given by Eq. (12.32) is
independent of the nature of the system Perfonning the Carnot cycle of
operations. Thus ‘Wwe are justified in using an ideal gas as a system in the
calculation of efficiency n of a Carnot engine. The ideal gas has a simple
equation of state, which allows us to readily calculate n, but the final result
for n, [Eq. (12.32)], is true for
any Carnot engine.
This final remark shows that in a Carnot
cycle,T; Baa (12.39)ia a universal relation
tndependent of the nature
of the syatem. Here 9, and Q, are reapectively,the
heat abaorbed and released iaothermally (from the hot and to the cold
reservoirs) in a Carnot engine. Equation (12.33), can, therefore,be used as a
relation to define a truly universal thermodynamic temperature scale that ia
independent of any particular properties of the
system used in the Carnot cycle. Ofcourse, for an
ideal gas as a working substance, this universal temperature is the same as the
ideal gas temperature introduced in section 12.11.
SUMMARY
1. The zeroth law of thermodynamics states that ‘two
systems tn thermal equilfbram with a third system are in thermal equilibrium
with each other. The Zeroth Law leads to the concept of temperature.
2. Internal energy of a system is the sum of kinetic
energies and potential energies of the molecular constituents of the system. It
does not inchide the over-all kinetic energy of the system. Heat and work are
two modes of energy transfer to the system. Heat is the
energy tranefer ariaing due te temperature
difference between the system and the surroundings, Work is energy transfer
brought about by other means, such as moving the piston of a cylinder containing
the gas, by raiaing or lowering some weight connected
to it.
3. The first law of thermodynamics is the general
law of conservation of energy applied to any system in which energy tranafer
from or to the surroundings (through heat and work} is taken into account. It
statea that
4Q =AU + AW where AQ ia the heat supplied to the
system, AW is the work done by the system and AU is the change in internal
energy of the system.
4. The apecific heat capacity of a substance is
defined by "1 AQ s=_>
m AT where m is the mass of the substance and AQ is
the heat required to change ite temperature by AT. The molar spectfic heat
capacity of a substance ta defined by cul Ag yeAT where jt is the number of
moles of the substance. For a solid, the law of equipartition of energy gtvea
C=3R which generally agrees with experiment at ordinary temperatures.Calorie is the old unit of heat.
1 calorie is the amount of heat required to raise the temperature of 1 g of
water from 14.6 °C to 15.6 °C. lcal = 4.186 J.
5. For an ideal gas, the molar specific heat
capacities at constant pressure and volume aatialy the relation c,-C, =R
where R is the universal gas constant.
6. Equilibrium states of a thermodynamic system are
deacribed by state variables. The value of a state variable depends only on the
particular state, not on the path used to arrive at that state. Examples of
state variables are preaaure (P), volume (V), temperature (T), and mass (m)}.
Heat and work are not state variables. An Equation of State (like
the ideal gas equation PV = jt RT) ia a relation
connecting different state variables.
7. A quasi-static process is an infinitely slow
procese such that the system remains in thermal and mechanical equilibrium with
the surroundings throughout. In a quasi-static process, the pressure and
temperature of the environment can differ from those of the system only
infinitesimally.
8. In an isothermal expansion of an ideal gas from
volume V, to V, at temperature T the heat absorbed (Q equals the work done (W)
by the gas, each given by . vy Q=We= zpRT Vy
9. In an adiabatic process of an ideal gas Pv"
= constant . C where y= C,
Work done by an ideal gas in an adiabatic change of
state from (P,, V,, T,) to (P,, V,, T,)ia wetRG-T) 7-1
10. Heat engine is a device in which a system undergoes
a cyclic process resulting in conversion of heat into work. If 9, 1s the heat
absorbed from the source, Q, is the heat released to the sink, and the work
output in one cycle is W, the efficiency 9 of the engine ia:no W 1. B 9; 9g:
11. Ina refrigerator or a heat pump, the system
extracts heat Q, from the cold reservoir and releases Q, amount of heat to the
hot reservotr, with work W done on the system. The co-efficient of performance
of a refrigerator is given by Qe Q» C= W Q91-Q2
12. The second law of thermodynamics disallows aome
processes conaistent with the First Law of Thermodynamics. It atates
Keluin-Planck statement No process ia possible whose sole result is the
absorption of heat from a reservoir and complete conversion of the heat into work.
Claustus sintement
No process is possible whose sole result ia the
transfer of heat from a colder object to a hotter object.Put aimply, the Second
Law implica that no heat engine can have efficiency y equal to
1 or no refrigerator can have co-efficient of
performance o equal to infinity.
15. A proceas is reveraible ff it can be reversed
such that both the system and the surroundings return to their original atates,
with no other change anywhere elae in the universe.
Spontaneous processes of nature are irreversible.
The idealised reveraible proceas is a quaai-static process with no diaeipative
factora such as friction, viecoaity, etc.
14, Carnot engine is a reveraible engine operating
between two temperatures T, (source) and T, (eink). The Carnot cycle consiste
of two isothermal processes commected by two adiabatic processes. The
efficiency of a Carnot engine is given by . 1 t
a= “T (Carnot engined)
No engine operating between two temperatures can
have efficiency greater than that of the Carnot engine.
16. If 9 > 0, heat ia added to the system If Q
<0, heat is removed to the system If W > 0, Work is done by the system If
W < 0, Work is done on the system
POINTS TO PONDER
1. Temperature of a body is related to its average
internal energy, not to the kmetic energy of motion of its centre of mass. A
bullet fired from a gun is not at a higher temperature because of its high
speed.
2. Equilibrium in thermodynamics refers to the
aituation when macroscopic variables describing the thermodynamic state of a
system do not depend on time. Equilibrtum of @ system in mechanics means the
uet external force and torque on the system are zero.
3. In a state of themmodynamic equilibrium, the
microscopic constituents of a system are not in equilibrium (in the sense of
mechanics).
4. Heat capacity, in general, depends on the process
the system goes through when heat is supplied.
5. In isothermal quasi-static processes, heat is
absorbed or gtven out by the system even though at every stage the gas has the
same temperature as that of the surrounding reservoir. This is possible because
of the tnfinttesimal difference in temperature between
the system and the reservotr.
EXERCISES
12.1 Ageyser heata water flowing at the rate of 3.0
litres per minute from 27 °C to 77 °C.If the geyser operates on a gas burner,
what is the rate of consumption of the fuel if ita heat of combustion is 4.0 x
10° J/g ?
12.2 What amount of heat must be supplied to 2.0 x
107 kg of nitrogen (at room temperature) to raise its temperature by 45 °C at
constant pressure ? (Molecular mass of N, = 28; R= 8.3 J mol" K".)
12.3 Explain why
(a) Two bodies at different temperatures T, and T,
if brought in thermal contact do not necessarily settle to the mean temperature
(T, + T,)/2.
(b) The coolant in a chemical or a nuclear plant
@1.c., the liquid used to prevent the different parte of a plant from getting
too hot) should have high specific heat.
(c) Air pressure in a car tyre increases during
driving.
(d) The climate of a harbour town is more temperate
than that of a town in a desert at the same latitude.
12.4 Acylinder with a movable piston contains 3
moles of hydrogen at standard temperature and pressure, The walls of the
cylinder are made of a heat insulator, and the piston ie insulated by having a
pile of sand on it. By what factor does the pressure of the gas increase if the
gas is compressed to half ite original volume 7?
12.5 In changing the state of a gas adiabatically
from an equilibrium state A to another equilibrium state B, an amount of work
equal to 22.3 J is done on the system. If the gas is taken from state A to B
via a process in which the net heat absorbed by the syatem is 9.35 cal, how
much ts the net work done by the system in the latter case 7
(Take 1 cal = 4.19 J)
12.8 Two cylinders A and B of equal capacity are
connected to each other via a stopcock.Acontaine a gas at standard temperature
and pressure. Bis completely evacuated.The entire system is thermally
insulated. The stopcock is suddenly opened. Answer the following :
a) What is the final pressure of the gas in A and B?
(b) What is the change in internal energy of the gas
?
(c) What je the change in the temperature of the gas
?
(d) Do the intermediate states of the system (before
settling to the final equilibrium atate) He on ite P-V-T surface ?
12.7 Aeteam engine delivers 5.4x10°J of work per
minute and services 3.6 x 10°J of heat per minute from its boiler. What is the
efficiency of the engine? How much heat is wasted per minute?
12.8 An electric heater supplies heat to a system at
a rate of 100W. If system performs work at a rate of 75 joules per second. At
what rate ia the internal energy increasing?
12.8 Athermodynemic system is taken from an original state to an intermediate state by the linear process shown in Fig. (12.13)
Ite
volume is then reduced to the original value from E to F by an isobaric
process.Calculate the total work done by the gas from D to E to F
12.10 A refrigerator ie to maintain eatables kept
inside at 9°C. If room temperature ta S6°C,calculate the coefficient of
performance.